DH 97MT2 receptor antagonist CAS# 220339-00-4 |
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
- Ellagic acid
Catalog No.:BCN5533
CAS No.:476-66-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 220339-00-4 | SDF | Download SDF |
| PubChem ID | 3467177 | Appearance | Powder |
| Formula | C22H26N2O | M.Wt | 334.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
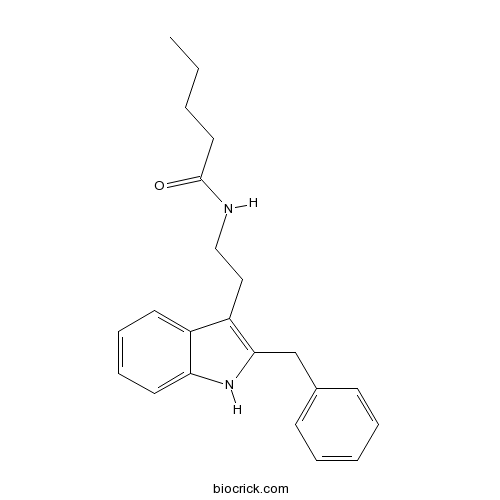
| Chemical Name | N-[2-(2-benzyl-1H-indol-3-yl)ethyl]pentanamide | ||
| SMILES | CCCCC(=O)NCCC1=C(NC2=CC=CC=C21)CC3=CC=CC=C3 | ||
| Standard InChIKey | HDOIPCLEKCEANF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H26N2O/c1-2-3-13-22(25)23-15-14-19-18-11-7-8-12-20(18)24-21(19)16-17-9-5-4-6-10-17/h4-12,24H,2-3,13-16H2,1H3,(H,23,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A relatively potent MT2 melatonin receptor antagonist (pKi value = 8.03), displaying 89- and 229-fold selectivity over MT1 and GPR50 (melatonin-related orphan receptor) respectively. |

DH 97 Dilution Calculator

DH 97 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9899 mL | 14.9495 mL | 29.8989 mL | 59.7979 mL | 74.7474 mL |
| 5 mM | 0.598 mL | 2.9899 mL | 5.9798 mL | 11.9596 mL | 14.9495 mL |
| 10 mM | 0.299 mL | 1.4949 mL | 2.9899 mL | 5.9798 mL | 7.4747 mL |
| 50 mM | 0.0598 mL | 0.299 mL | 0.598 mL | 1.196 mL | 1.4949 mL |
| 100 mM | 0.0299 mL | 0.1495 mL | 0.299 mL | 0.598 mL | 0.7475 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- TRO 19622
Catalog No.:BCC5288
CAS No.:22033-87-0
- 3,11,12-Trihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1487
CAS No.:220328-04-1
- 5-[2-[Tert-butyl(dimethyl)silyl]oxyethyl]-2,2-dimethyl-3a,6a-dihydrofuro[2,3-d][1,3]dioxol-6-one
Catalog No.:BCC8592
CAS No.:220328-03-0
- Cinnamamide
Catalog No.:BCN4942
CAS No.:22031-64-7
- Triptohairic acid
Catalog No.:BCN8060
CAS No.:220209-71-2
- O-Methyldauricine
Catalog No.:BCC8225
CAS No.:2202-17-7
- Eichlerialactone
Catalog No.:BCN4941
CAS No.:2202-01-9
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Vincetoxicoside B
Catalog No.:BCN2864
CAS No.:22007-72-3
- Nu 6027
Catalog No.:BCC1154
CAS No.:220036-08-8
- Ixabepilone
Catalog No.:BCC1666
CAS No.:219989-84-1
- Fmoc-β-Homo-Tyr(tBu)-OH
Catalog No.:BCC2621
CAS No.:219967-69-8
- 5-Epicanadensene
Catalog No.:BCN7349
CAS No.:220384-17-8
- ShK-Dap22
Catalog No.:BCC5990
CAS No.:220384-25-8
- Polycephalin C
Catalog No.:BCN1852
CAS No.:220422-37-7
- Kaempferol 5-methyl ether
Catalog No.:BCN3426
CAS No.:22044-80-0
- Leptomerine
Catalog No.:BCN1486
CAS No.:22048-97-1
- 1-Methyl-2-pentyl-4(1H)-quinolinone
Catalog No.:BCN4943
CAS No.:22048-98-2
- Caspase-3/7 Inhibitor I
Catalog No.:BCC1140
CAS No.:220509-74-0
- Diphyllin
Catalog No.:BCN8066
CAS No.:22055-22-7
- GB 1a
Catalog No.:BCN7237
CAS No.:220611-41-6
- Tigecycline
Catalog No.:BCC2499
CAS No.:220620-09-7
- RJR-2403 oxalate
Catalog No.:BCC1902
CAS No.:220662-95-3
- Antisauvagine-30
Catalog No.:BCC5868
CAS No.:220673-95-0
Oral BG-12 (dimethyl fumarate) for relapsing-remitting multiple sclerosis: a review of DEFINE and CONFIRM. Evaluation of: Gold R, Kappos L, Arnold D, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012;367:1098-107; and Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087-97.[Pubmed:23971970]
Expert Opin Pharmacother. 2013 Oct;14(15):2145-56.
INTRODUCTION: Multiple sclerosis (MS) is an autoimmune neurodegenerative disease of the central nervous system involving inflammation, chronic demyelination and axonal loss. MS affects more than 2 million people worldwide. AREAS COVERED: This article aims to summarize the findings from two pivotal 2-year, randomized, double-blind, placebo-controlled, Phase III studies of BG-12 (dimethyl fumarate) for relapsing-remitting MS (RRMS): DEFINE (Determination of the Efficacy and Safety of Oral Fumarate in RRMS) and CONFIRM (Comparator and an Oral Fumarate in RRMS). Results from both studies demonstrated that BG-12 provides clinical and radiological efficacy over 2 years across a range of outcomes. These results were apparent as early as 12 weeks and sustained over the course of both studies. BG-12 was found to have an acceptable safety profile, with a similar overall incidence of adverse events across all treatment groups. EXPERT OPINION: The combination of robust efficacy, ease of administration and established safety profile is unique to a new therapy in MS. Findings from the pivotal Phase III studies support BG-12 as a potential initial oral treatment for patients with RRMS or as an alternative to other currently available therapies.
Molecular and pharmacological evidence for MT1 melatonin receptor subtype in the tail artery of juvenile Wistar rats.[Pubmed:10433507]
Br J Pharmacol. 1999 Jun;127(4):987-95.
1. In this study reverse transcriptase-polymerase chain reaction (RT-PCR) has been used to identify mt1 and MT2 receptor mRNA expression in the rat tail artery. The contributions of both receptors to the functional response to melatonin were examined with the putative selective MT2 receptor antagonists, 4-phenyl-2-propionamidotetraline (4-P-PDOT) and 2-benzyl-N-pentanoyltryptamine. In addition, the action of melatonin on the second messenger cyclic AMP was investigated. 2. Using RT-PCR, mt1 receptor mRNA was detected in the tail artery from seven rats. In contrast MT2 receptor mRNA was not detected even after nested PCR. 3. At low concentrations of the MT2 selective ligands, neither 10 nM 4-P-PDOT (pEC50=8.70+/-0.31 (control) vs 8.73+/-0.16, n=6) nor 60 nM 2-benzyl-NV-pentanoyltryptamine (pEC50= 8.53+/-0.20 (control) vs 8.83+/-0.38, n = 6) significantly altered the potency of melatonin in the rat tail artery. 4. At concentrations non-selective for mt1 and MT2 receptors. 4-P-PDOT (3 microM) and 2-benzyl-N-pentanoyltryptamine (5 microM) caused a significant rightward displacement of the vasoconstrictor effect of melatonin. In the case of 4-P-PDOT, the estimated pKB (6.17+/-0.16, n=8) is similar to the binding affinity for mt1 receptor. 5. Pre-incubation with 1 microM melatonin did not affect the conversion of [3H]-adenine to [3H]-cyclic AMP under basal condition (0.95+/-0.19% conversion (control) vs 0.92+/-0.19%, n=4) or following exposure to 30 microM forskolin (5.20+/-1.30% conversion (control) vs 5.35+/-0.90%, n=4). 6. Based on the above findings, we conclude that melatonin receptor on the tail artery belongs to the MT1 receptor subtype, and that this receptor is probably independent of the adenylyl cyclase pathway.
Comparison of the structure-activity relationships of melatonin receptor agonists and antagonists: lengthening the N-acyl side-chain has differing effects on potency on Xenopus melanophores.[Pubmed:9840420]
Naunyn Schmiedebergs Arch Pharmacol. 1998 Nov;358(5):522-8.
The potency and affinity of two series of melatonin receptor ligands were examined using the pigment aggregation response in a clonal line of Xenopus laevis melanophores and radioligand binding assays on native receptors in chicken brain, recombinant human mt1 and MT2 and Xenopus laevis mel1c receptor subtypes. One series was based on melatonin and had a methoxy group at the 5-position of the indole ring, while the other was based on luzindole and lacked this substituent but did have a 2-benzyl moiety; the N-acyl group of each series of analogues was varied from one to five carbon atoms. All analogues in the melatonin series were full agonists in melanophores (pEC50 7.76-10.24), while all compounds in the luzindole series were competitive melatonin antagonists (pA2 5.47-6.60). With the agonist series, increasing the N-acyl side-chain from one to three carbon atoms was well tolerated in both the functional and binding assays, but further lengthening of the side-chain progressively and dramatically reduced potency and affinity. In contrast, for the antagonist series neither potency nor binding affinity changed substantially with the length of the N-acyl chain, except at the recombinant MT2 subtype where two of the analogues had a lower affinity. In binding assays, three of the five antagonists were MT2-selective; the most selective analogue (N-pentanoyl 2-benzyltryptamine, MT2 pKi 8.03) having 89- and 229-fold higher affinity than at mt1 or mel1c receptor subtypes. The different structure-activity relationships of these receptor agonists and antagonists is discussed with regard to the possible binding sites of agonists and antagonists within the receptor protein.


