KetoprofenCAS# 22071-15-4 |
- PF-4981517
Catalog No.:BCC2270
CAS No.:1390637-82-7
- Abiraterone
Catalog No.:BCC2259
CAS No.:154229-19-3
- Avasimibe
Catalog No.:BCC2274
CAS No.:166518-60-1
- Alizarin
Catalog No.:BCN3479
CAS No.:72-48-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 22071-15-4 | SDF | Download SDF |
| PubChem ID | 3825 | Appearance | Powder |
| Formula | C16H14O3 | M.Wt | 254.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (393.27 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
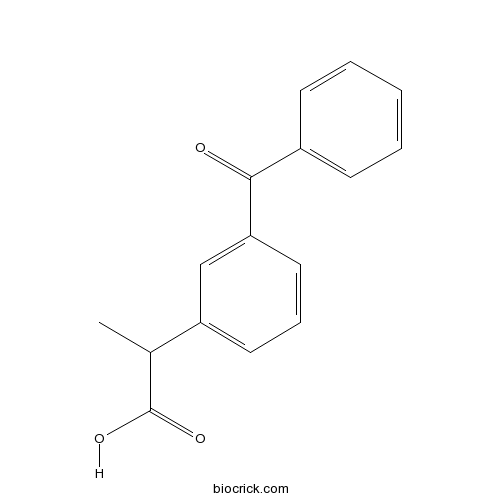
| Chemical Name | 2-(3-benzoylphenyl)propanoic acid | ||
| SMILES | CC(C1=CC=CC(=C1)C(=O)C2=CC=CC=C2)C(=O)O | ||
| Standard InChIKey | DKYWVDODHFEZIM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H14O3/c1-11(16(18)19)13-8-5-9-14(10-13)15(17)12-6-3-2-4-7-12/h2-11H,1H3,(H,18,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ketoprofen (Actron) is a non-selective NSAID with IC50 of 0.5 μM and 2.33 μM for human recombinant COX-1 and COX-2, respectively.
Target: COX
Ketoprofen is one of the propionic acid class of nonsteroidal anti-inflammatory drug (NSAID) with analgesic and antipyretic effects [1]. It acts by inhibiting the body's production of prostaglandin.
Ketoprofen 50 and 100 mg and of paracetamol with diclofenac were associated with lower pain scores than paracetamol alone. In the study of paracetamol and ketoprofen, the combination reduced pain scores both at rest and on movement after disc surgery compared with paracetamol alone. In a study involving spinal fusion surgery, the combination of propacetamol and ketoprofen 100 mg improved pain scores assessed by VAS pain intensity differences [2]. References: | |||||

Ketoprofen Dilution Calculator

Ketoprofen Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9327 mL | 19.6634 mL | 39.3267 mL | 78.6535 mL | 98.3168 mL |
| 5 mM | 0.7865 mL | 3.9327 mL | 7.8653 mL | 15.7307 mL | 19.6634 mL |
| 10 mM | 0.3933 mL | 1.9663 mL | 3.9327 mL | 7.8653 mL | 9.8317 mL |
| 50 mM | 0.0787 mL | 0.3933 mL | 0.7865 mL | 1.5731 mL | 1.9663 mL |
| 100 mM | 0.0393 mL | 0.1966 mL | 0.3933 mL | 0.7865 mL | 0.9832 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ketoprofen (Actron) is a non-selective NSAID with IC50 of 0.5 μM and 2.33 μM for human recombinant COX-1 and COX-2, respectively.
- Oxonic acid potassium salt
Catalog No.:BCC4165
CAS No.:2207-75-2
- Antisauvagine-30
Catalog No.:BCC5868
CAS No.:220673-95-0
- RJR-2403 oxalate
Catalog No.:BCC1902
CAS No.:220662-95-3
- Tigecycline
Catalog No.:BCC2499
CAS No.:220620-09-7
- GB 1a
Catalog No.:BCN7237
CAS No.:220611-41-6
- Diphyllin
Catalog No.:BCN8066
CAS No.:22055-22-7
- Caspase-3/7 Inhibitor I
Catalog No.:BCC1140
CAS No.:220509-74-0
- 1-Methyl-2-pentyl-4(1H)-quinolinone
Catalog No.:BCN4943
CAS No.:22048-98-2
- Leptomerine
Catalog No.:BCN1486
CAS No.:22048-97-1
- Kaempferol 5-methyl ether
Catalog No.:BCN3426
CAS No.:22044-80-0
- Polycephalin C
Catalog No.:BCN1852
CAS No.:220422-37-7
- ShK-Dap22
Catalog No.:BCC5990
CAS No.:220384-25-8
- Curlignan
Catalog No.:BCN3977
CAS No.:220736-54-9
- Aquifoliunine E-III
Catalog No.:BCN3096
CAS No.:220751-20-2
- Aminopurvalanol A
Catalog No.:BCC7249
CAS No.:220792-57-4
- NG,NG-Dimethylarginine dihydrochloride
Catalog No.:BCN1485
CAS No.:220805-22-1
- 7-Methoxy-8-Hydroxy-4-Methylcoumarin
Catalog No.:BCC8291
CAS No.:22084-94-2
- Thunalbene
Catalog No.:BCN3688
CAS No.:220862-05-5
- 2,3-O-Isopropylidenyl euscaphic acid
Catalog No.:BCN4944
CAS No.:220880-90-0
- GW5074
Catalog No.:BCC4391
CAS No.:220904-83-6
- 13-O-Cinnamoylbaccatin III
Catalog No.:BCN7344
CAS No.:220932-65-0
- 13-O-Deacetyltaxumairol Z
Catalog No.:BCN4945
CAS No.:220935-39-7
- Antalarmin hydrochloride
Catalog No.:BCC7480
CAS No.:220953-69-5
- Lumiracoxib
Catalog No.:BCC4440
CAS No.:220991-20-8
Chemical Modifications of Ketoprofen (NSAID) in Search of Better Lead Compounds: A Review of Literature From 2004-2016.[Pubmed:28215167]
Antiinflamm Antiallergy Agents Med Chem. 2017;15(3):154-177.
BACKGROUND: Ketoprofen, a potent anti-inflammatory, analgesic and anti-pyretic drug belonging to the propionic acid class was synthesized in 1968. Rapid absorption, simple metabolism, faster blood brain barrier crossing and high antinociceptive activity are the features responsible for its high use. But, free acidic moiety present in its structure is the major factor that declines its popularity by causing various gastric side effects. Many researchers have chemically modified this drug with the aim to discover an improved and safe NSAID candidate or a new drug with altered activity. We thoroughly searched the literature and found that during the period 2004-2016, more than fifty reports are available on chemical modification of Ketoprofen. Along with this, many patents involving chemical modification of Ketoprofen have also been reported. However, it was very surprising to note that there are only a few review articles available covering only its pharmacological and clinical properties. There is no review article available covering the chemistry part of Ketoprofen. This motivated us to compile the information available on Ketoprofen and its derivatives. The purpose of this article is to present an updated review about this topic. METHODS: We thoroughly searched the peer reviewed research literature and compiled all such reportings (2004 onwards) for the benefit of researchers who further want to work on Ketoprofen or other NSAIDs. RESULTS: Studies have been conducted to invent strategies to reduce the ulcerogenic properties of Ketoprofen and in the course of time, its modified and improved derivatives have been synthesized in search of safer NSAIDs. Along with the aim of reducing the gastric side-effects, researchers have also done chemical modifications in the structure of Ketoprofen to improve its solubility, to alter its blood brain-barrierr permeability, to improve its pharmacodynamic profile and to get derivatives possessing antioxidant, antiviral, anticancer and immunomodulatory activities. CONCLUSION: The findings of the review confirm that chemical modifications of Ketoprofen decrease ulcer producing side effect while maintaining its desirable actions. Some derivatives were also found to possess better activity profile compared to the parent drug.
Ketoprofen Microemulsion for Improved Skin Delivery and In Vivo Anti-inflammatory Effect.[Pubmed:28374340]
AAPS PharmSciTech. 2017 Oct;18(7):2783-2791.
We have designed a microemulsion (ME) containing Ketoprofen (KET) for anti-inflammatory effect evaluated using the rat paw edema model. The ME was prepared by adding propylene glycol (PG) loaded with 1% KET/water (3:1, w/w), to a mixture of sorbitan monooleate and polysorbate 80 (47.0%) at 3:1 (w/w) and canola oil (38.0%). The physicochemical characterization of KET-loaded ME involved particle size and zeta potential determination, entrapment efficiency, calorimetric analysis, and in vitro drug release. The in vivo anti-inflammatory study employed male Wistar rats. Measurement of the foot volume was performed using a caliper immediately before and 2, 4, and 6 h after injection of Aerosil. KET-loaded ME showed particle size around 20 nm, with zeta potential at -16 mV and entrapment efficiency at 70%. Moreover, KET was converted to the amorphous state when loaded in the formulation and it was shown that the drug was slowly released from the ME. Finally, the in vivo biological activity was similar to that of the commercial gel, but ME better controlled edema at 4 h. These results demonstrated that the ME formulation is an alternative strategy for improving KET skin permeation for anti-inflammatory effect. Furthermore, our findings are promising considering that the developed ME was loaded with only 1% KET, and the formulation was able to keep a similar release profile and in vivo effect compared to the commercial gel with 2.5% KET. Therefore, the KET-loaded developed herein ME is likely to have a decreased side effect compared with that of the commercial gel, but both presented the same efficacy.
Substance P expression in the gingival tissue after upper third molar extraction: effect of ketoprofen, a preliminary study.[Pubmed:28337899]
J Biol Regul Homeost Agents. 2017 Jan-Mar;31(1):239-244.
The aim of this study was to evaluate substance P (SP) levels and the effect of a non-steroidal anti-inflammatory drug (NSAID), Ketoprofen, on SP in the pericoronal gingival tissue after extraction of upper third molars. A sample of 20 young non-smoking systemically healthy adults of both sexes, with a healthy upper third molar to extract for orthodontic purposes, was selected. After extraction, a sample of the gingival tissue of the pericoronal region was collected with a sterile scalpel, placed into test tubes and kept frozen at -20 degrees C until the SP determination. SP levels were determined by using a commercially available enzyme immunoassay (ELISA) kit. The subjects were randomly divided into two groups: group 1 received a single dose of Ketoprofen 30 minutes prior to the experimental procedure. The subjects of group 2 did not receive any kind of drug administration before extraction. The patients were asked to complete a diary on the postoperative pain. A relevant amount of SP was measured in all the gingival samples. No statistically significant difference could be detected in SP expression between the two groups. In group 1 pain appearance was significantly delayed (6.2+/-0.13 hours) in comparison with group 2 (3.95+/-0.2 hours). In this small selected group of subjects and limited study design, preventive administration of Ketoprofen did not significantly affect the gingival levels of SP, the clinical recommendation emerging is that of NSAID administration postoperatively but before pain appearance in order to optimize the management of pain of the patient.
Peptide dendrimer-conjugates of ketoprofen: Synthesis and ex vivo and in vivo evaluations of passive diffusion, sonophoresis and iontophoresis for skin delivery.[Pubmed:28285173]
Eur J Pharm Sci. 2017 May 1;102:237-249.
The aim of this study was to evaluate skin delivery of Ketoprofen when covalently tethered to mildly cationic (2(+) or 4(+)) peptide dendrimers prepared wholly by solid phase peptide synthesis. The amino acids glycine, arginine and lysine formed the dendrimer with Ketoprofen tethered either to the lysine side-arm (Nepsilon) or periphery of dendrimeric branches. Passive diffusion, sonophoresis- and iontophoresis-assisted permeation of each peptide dendrimer-drug conjugate (D1-D4) was studied across mouse skin, both in vitro and in vivo. In addition, skin toxicity of dendrimeric conjugates when trialed with iontophoresis or sonophoresis was also evaluated. All dendrimeric conjugates improved aqueous solubility at least 5-fold, compared to Ketoprofen alone, while also exhibiting appreciable lipophilicity. In vitro passive diffusion studies revealed that Ketoprofen in its native form was delivered to a greater extent, compared with a dendrimer-conjugated form at the end of 24h (Q24h (mug/cm(2)): Ketoprofen (68.06+/-3.62)>D2 (49.62+/-2.92)>D4 (19.20+/-0.89)>D1 (6.45+/-0.40)>D3 (2.21+/-0.19). However, sonophoresis substantially increased the skin permeation of Ketoprofen-dendrimer conjugates in 30min (Q30min (mug/cm(2)): D4 (122.19+/-7.14)>D2 (66.74+/-3.86)>D1 (52.10+/-3.22)>D3 (41.66+/-3.22)) although Ketoprofen alone again proved superior (Q30min: 167.99+/-9.11mug/cm(2)). Next, application of iontophoresis was trialed and shown to considerably increase permeation of dendrimeric Ketoprofen in 6h (Q6h (mug/cm(2)): D2 (711.49+/-39.14)>D4 (341.23+/-16.43)>D3 (89.50+/-4.99)>D1 (50.91+/-2.98), with a Q6h value of 96.60+/-5.12mug/cm(2) for Ketoprofen alone). In vivo studies indicated that therapeutically relevant concentrations of Ketoprofen could be delivered transdermally when iontophoresis was paired with D2 (985.49+/-43.25ng/mL). Further, histopathological analysis showed that the dendrimeric approach was a safe mode as Ketoprofen alone. The present study successfully demonstrates that peptide dendrimer conjugates of Ketoprofen, when combined with non-invasive modalities, such as iontophoresis can enhance skin permeation with clinically relevant concentrations achieved transdermally.


