GlucotropaeolinCAS# 5115-71-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5115-71-9 | SDF | Download SDF |
| PubChem ID | 70702337 | Appearance | Beige powder |
| Formula | C14H18KNO9S2 | M.Wt | 447.5 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | Benzylglucosinolate potassium salt | ||
| Solubility | Soluble in methanol and water; slightly soluble in ethan | ||
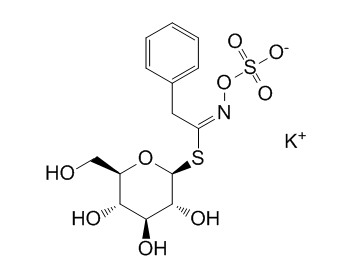
| Chemical Name | potassium;[(E)-[2-phenyl-1-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]sulfanylethylidene]amino] sulfate | ||
| SMILES | C1=CC=C(C=C1)CC(=NOS(=O)(=O)[O-])SC2C(C(C(C(O2)CO)O)O)O.[K+] | ||
| Standard InChIKey | UYCWNAZWHVREMO-GYVLLFFHSA-M | ||
| Standard InChI | InChI=1S/C14H19NO9S2.K/c16-7-9-11(17)12(18)13(19)14(23-9)25-10(15-24-26(20,21)22)6-8-4-2-1-3-5-8;/h1-5,9,11-14,16-19H,6-7H2,(H,20,21,22);/q;+1/p-1/b15-10+; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Glucotropaeolin Dilution Calculator

Glucotropaeolin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2346 mL | 11.1732 mL | 22.3464 mL | 44.6927 mL | 55.8659 mL |
| 5 mM | 0.4469 mL | 2.2346 mL | 4.4693 mL | 8.9385 mL | 11.1732 mL |
| 10 mM | 0.2235 mL | 1.1173 mL | 2.2346 mL | 4.4693 mL | 5.5866 mL |
| 50 mM | 0.0447 mL | 0.2235 mL | 0.4469 mL | 0.8939 mL | 1.1173 mL |
| 100 mM | 0.0223 mL | 0.1117 mL | 0.2235 mL | 0.4469 mL | 0.5587 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Glucoerucin
Catalog No.:BCN8977
CAS No.:15592-37-7
- Glucoberteroin
Catalog No.:BCN8976
CAS No.:245550-65-6
- Glucoiberin
Catalog No.:BCN8975
CAS No.:15592-34-4
- Glucobrassicanapin
Catalog No.:BCN8974
CAS No.:245550-58-7
- Sinalbin
Catalog No.:BCN8973
CAS No.:20196-67-2
- Glucocapparin
Catalog No.:BCN8972
CAS No.:15592-33-3
- Glucolimnanthin
Catalog No.:BCN8971
CAS No.:111810-95-8
- Glucobarbarin
Catalog No.:BCN8970
CAS No.:21087-78-5
- Phyllalbine
Catalog No.:BCN8969
CAS No.:4540-25-4
- Epiprogoitrin
Catalog No.:BCN8968
CAS No.:21087-74-1
- 4-Hydroxyglucobrassicin
Catalog No.:BCN8967
CAS No.:83327-20-2
- 4-Methoxyglucobrassicin
Catalog No.:BCN8966
CAS No.:83327-21-3
- Glucomoringin
Catalog No.:BCN8979
CAS No.:316165-49-8
- Lupinine
Catalog No.:BCN8981
CAS No.:486-70-4
- Glucoalyssin
Catalog No.:BCN8982
CAS No.:499-37-6
- Neoglucobrassicin potassium salt
Catalog No.:BCN8983
CAS No.:5187-84-8
- Gluconapin
Catalog No.:BCN8984
CAS No.:245550-57-6
- Progoitrin
Catalog No.:BCN8985
CAS No.:21087-77-4
- 18-Hydroxyspartioidine
Catalog No.:BCN8941
CAS No.:
- Heliotridine N-oxide
Catalog No.:BCN8944
CAS No.:
- Rinderine hydrochloride
Catalog No.:BCN8947
CAS No.:26131-12-4
- Jacoline N-oxide
Catalog No.:BCN8954
CAS No.:
- Merepoxine N-oxide
Catalog No.:BCN8956
CAS No.:
- 11-(Methylsulfinyl)undecylglucosinolate
Catalog No.:BCN8980
CAS No.:
Bunias erucago L.: Glucosinolate Profile and In Vitro Biological Potential.[Pubmed:30791395]
Molecules. 2019 Feb 19;24(4). pii: molecules24040741.
Bunias erucago belongs to the Brassicaceae family, which represents a forgotten crop of the Euro-Mediterranean area. The aim of the present study was to determine the glucosinolate profile in different plant parts and biological properties (antioxidant, anticholinesterase, and cytotoxic activities) of the isolates containing glucosinolate breakdown products. The chemical profiles were determined by using HPLC-PDA-MS/MS of desulfoglucosinolates and GC-MS of glucosinolate degradation products. The analysis of B. erucago showed the presence of seven glucosinolates: gluconapin (1), glucoraphasatin (2), glucoraphenin (3), glucoerucin (4), glucoraphanin (5), Glucotropaeolin (6), and glucosinalbin (7). The total glucosinolate content ranged from 7.0 to 14.6 micromol/g of dry weight, with the major glucosinolate glucosinalbin in all parts. The antioxidant activity of all volatile isolates was not notable. At a tested concentration of 227 mug/mL, flower hydro-distillate (FH) showed good AChE inhibition, i.e., 40.9%, while root hydro-distillate (RH) had good activity against BChE, i.e., 54.3%. FH showed the best activity against both tested human bladder cancer cell lines, i.e., against T24 after 72 h, which have IC50 of 16.0 mug/mL, and against TCCSUP after 48 h with IC50 of 7.8 mug/mL, and can be considered as highly active. On the other hand, RH showed weak activity against tested cancer cells.
Antimicrobial and Cytotoxic Activities of Lepidium latifolium L. Hydrodistillate, Extract and Its Major Sulfur Volatile Allyl Isothiocyanate.[Pubmed:30714673]
Chem Biodivers. 2019 Apr;16(4):e1800661.
The cultivated Lepidium latifolium L. was investigated to decipher its glucosinolate profile, antimicrobial, and cytotoxic activities. HPLC/ESI-MS analyses of the intact glucosinolates and GC/MS analysis of their hydrolysis products showed the presence of sinigrin (1), glucocochlearin (2), Glucotropaeolin (3), and 4-methoxyglucobrassicin (4). Hydrodistillate, extract, and allyl isothiocyanate, the main volatile resulting from sinigrin degradation, showed antimicrobial activity against all eleven tested pathogenic and food spoilage bacteria and fungi, with highest effect observed against Candida albicans with MIC50 8 and 16 mug/mL. Hydrodistillate and extract showed the best cytotoxic activity on bladder cancer UM-UC-3 cell line during an incubation time of 24 h (IC50 192.9 and 133.8 mug/mL, respectively), while the best effect on glioblastoma LN229 cell line was observed after 48 h (IC50 110.8 and 30.9 mug/mL, respectively). Pure allyl isothiocyanate displayed a similar trend in cytotoxic effect on both cell lines (IC50 23.3 and 36.5 mug/mL after 24 h and 48 h, respectively).
Comparative analysis of glucosinolates and metabolite profiling of green and red mustard (brassica juncea) hairy roots.[Pubmed:30148032]
3 Biotech. 2018 Sep;8(9):382.
Here, accumulation of glucosinolates and expression of glucosinolates biosynthesis genes in green and red mustard hairy roots were identified and quantified by HPLC and qRT-PCR analyses. The total glucosinolates content of green mustard hairy root (10.09 microg/g dry weight) was 3.88 times higher than that of red mustard hairy root. Indolic glucosinolates (glucobrassicin, 4-methoxyglucobrassicin, and neoglucobrassicin) in green mustard were found at 30.92, 6.95, and 5.29 times higher than in red mustard hairy root, respectively. Conversely, levels of Glucotropaeolin (aromatic glucosinolate) was significantly higher in red mustard than in green mustard. Accumulation of glucoraphasatin, an aliphatic glucosinolate, was only observed only in red mustard hairy roots. Quantitative real-time PCR analysis showed that the expression level of genes related to aliphatic and aromatic glucosinolate biosynthesis were higher in red mustard, exception BjCYP83B. The expression of BjCYP79B2, which encodes a key enzyme involved in the indolic glucosinolate biosynthetic pathway, was higher in green mustard than in red mustard. Additionally, to further distinguish between green mustard and red mustard hairy roots, hydrophilic and lipophilic compounds were identified by gas chromatography-mass spectrometry and subjected to principal component analysis. The results indicated that core primary metabolites and glucosinolate levels were higher in the hairy roots of green mustard than in those of red mustard.
Oral administration of nasturtium affects peptide YY secretion in male subjects.[Pubmed:28371338]
Mol Nutr Food Res. 2017 Aug;61(8).
SCOPE: Nasturtium plants contain the glucosinolate Glucotropaeolin and its corresponding breakdown product benzyl isothiocyanate (BITC), the latter being intensively studied with regard to cancer chemoprevention and anti-inflammatory properties. In addition, recent research has shown that isothiocyanates are able to activate the release of several gut hormones in vitro and in rodent studies. Here, we tested the effects of a dietary nasturtium administration on circulating levels of gut hormones in humans. METHODS AND RESULTS: Metabolically healthy males (n = 15) received a single oral dose of 10 g freeze-dried nasturtium leaf material suspended in water or only water (control). Blood samples were taken every hour and serum concentrations of insulin, C-peptide, glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic peptide (GIP), and peptide (PYY) were analyzed. Oral nasturtium intake resulted in an increased release of PYY over a time period of 6 h whereas circulating levels of other hormones were not changed. CONCLUSION: Given the finding that nasturtium consumption enhances secretion of PYY, a key hormone involved in energy regulation, special diets containing nasturtium, or supplementation with nasturtium or BITC might be considered in the treatment of obesity.
Development of an efficient glucosinolate extraction method.[Pubmed:28344636]
Plant Methods. 2017 Mar 21;13:17.
BACKGROUND: Glucosinolates, anionic sulfur rich secondary metabolites, have been extensively studied because of their occurrence in the agriculturally important brassicaceae and their impact on human and animal health. There is also increasing interest in the biofumigant properties of toxic glucosinolate hydrolysis products as a method to control agricultural pests. Evaluating biofumigation potential requires rapid and accurate quantification of glucosinolates, but current commonly used methods of extraction prior to analysis involve a number of time consuming and hazardous steps; this study aimed to develop an improved method for glucosinolate extraction. RESULTS: Three methods previously used to extract glucosinolates from brassicaceae tissues, namely extraction in cold methanol, extraction in boiling methanol, and extraction in boiling water were compared across tissue type (root, stem leaf) and four brassicaceae species (B. juncea, S. alba, R. sativus, and E. sativa). Cold methanol extraction was shown to perform as well or better than all other tested methods for extraction of glucosinolates with the exception of glucoraphasatin in R. sativus shoots. It was also demonstrated that lyophilisation methods, routinely used during extraction to allow tissue disruption, can reduce final glucosinolate concentrations and that extracting from frozen wet tissue samples in cold 80% methanol is more effective. CONCLUSIONS: We present a simplified method for extracting glucosinolates from plant tissues which does not require the use of a freeze drier or boiling methanol, and is therefore less hazardous, and more time and cost effective. The presented method has been shown to have comparable or improved glucosinolate extraction efficiency relative to the commonly used ISO method for major glucosinolates in the Brassicaceae species studied: sinigrin and gluconasturtiin in B. juncea; sinalbin, Glucotropaeolin, and gluconasturtiin in S. alba; glucoraphenin and glucoraphasatin in R. sativus; and glucosatavin, glucoerucin and glucoraphanin in E. sativa.
Identification of Glucosinolates in Seeds of Three Brassicaceae Species Known to Hyperaccumulate Heavy Metals.[Pubmed:27981800]
Chem Biodivers. 2017 Mar;14(3).
Plants from the Brassicaceae family are known to contain secondary metabolites called glucosinolates. Our goal was to establish by LC/MS the glucosinolate profile of seeds of three Brassicaceae species known to hyperaccumulate heavy metals. We investigated Alyssum fallacinum auct. non Hausskn., Iberis intermedia Guers., and Noccaea caerulescens (J. Presl & C. Presl) F. K. Mey. Our results indicate that A. fallacinum seeds contain glucoiberin and glucoibervirin, which had not been previously identified in this plant. Furthermore, we report for the first time the presence of glucoiberin, glucoibervirin, Glucotropaeolin, and sinigrin in I. intermedia. We have detected for the first time glucoconringiin in N. caerulescens. In addition, glucosinalbin, 4-hydroxyglucobrassicin, and glucomoringin were also detected.
Synthesis and spectral characterization of 2,2-diphenylethyl glucosinolate and HPLC-based reaction progress curve data for the enzymatic hydrolysis of glucosinolates by Sinapis alba myrosinase.[Pubmed:27981206]
Data Brief. 2016 Nov 28;10:151-181.
The data presented in this article are related to the research article, "HPLC-based enzyme kinetics assay for glucosinolate hydrolysis facilitate analysis of systems with both multiple reaction products and thermal enzyme denaturation" (C.K. Klingaman, M.J. Wagner, J.R. Brown, J.B. Klecker, E.H. Pauley, C.J. Noldner, J.R. Mays,) [1]. This data article describes (1) the synthesis and spectral characterization data of a non-natural glucosinolate analogue, 2,2-diphenylethyl glucosinolate, (2) HPLC standardization data for glucosinolate, isothiocyanate, nitrile, and amine analytes, (3) reaction progress curve data for enzymatic hydrolysis reactions with variable substrate concentration, enzyme concentration, buffer pH, and temperature, and (4) normalized initial velocities of hydrolysis/formation for analytes. These data provide a comprehensive description of the enzyme-catalyzed hydrolysis of 2,2-diphenylethyl glucosinolate (5) and Glucotropaeolin (6) under widely varied conditions.
HPLC-based kinetics assay facilitates analysis of systems with multiple reaction products and thermal enzyme denaturation.[Pubmed:27742213]
Anal Biochem. 2017 Jan 1;516:37-47.
Glucosinolates are plant secondary metabolites abundant in Brassica vegetables that are substrates for the enzyme myrosinase, a thioglucoside hydrolase. Enzyme-mediated hydrolysis of glucosinolates forms several organic products, including isothiocyanates (ITCs) that have been explored for their beneficial effects in humans. Myrosinase has been shown to be tolerant of non-natural glucosinolates, such as 2,2-diphenylethyl glucosinolate, and can facilitate their conversion to non-natural ITCs, some of which are leads for drug development. An HPLC-based method capable of analyzing this transformation for non-natural systems has been described. This current study describes (1) the Michaelis-Menten characterization of 2,2-diphenyethyl glucosinolate and (2) a parallel evaluation of this analogue and the natural analogue Glucotropaeolin to evaluate effects of pH and temperature on rates of hydrolysis and product(s) formed. Methods described in this study provide the ability to simultaneously and independently analyze the kinetics of multiple reaction components. An unintended outcome of this work was the development of a modified Lambert W(x) which includes a parameter to account for the thermal denaturation of enzyme. The results of this study demonstrate that the action of Sinapis alba myrosinase on natural and non-natural glucosinolates is consistent under the explored range of experimental conditions and in relation to previous accounts.
Glucosinolate and Desulfo-glucosinolate Metabolism by a Selection of Human Gut Bacteria.[Pubmed:27301252]
Curr Microbiol. 2016 Sep;73(3):442-451.
Glucosinolate (GSL) hydrolysis is mediated by the enzyme myrosinase which together with specifier proteins can give rise to isothiocyanates (ITCs), thiocyanates, and nitriles (NITs) in cruciferous plants. However, little is known about the metabolism of GSLs by the human gut flora. The aim of the work was to investigate the metabolic fates of sinigrin (SNG), Glucotropaeolin (GTP), gluconasturtiin (GNT), and their corresponding desulfo-GSLs (DS-GSLs). Three human gut bacterial strains, Enterococcus casseliflavus CP1, Lactobacillus agilis R16, and Escherichia coli VL8, were chosen for this study. GNT was metabolized to completion within 24 h to phenethyl ITC and phenethyl NIT (PNIT) by all bacteria, except for L. agilis R16 which produced only PNIT. At least 80 % of GTP and SNG were metabolized by all bacteria within 24 h to the corresponding ITCs and NITs. The pH of media over time gradually became acidic for both L. agilis R16 and E. coli VL8, while for E. casseliflavus CP1 the media became slightly alkaline with NIT and ITC production occurring between pH 3.0 and 7.5. ITC production peaked between 4 and 10 h in most cases and gradually declined while NIT production increased and remained relatively constant over time. The total percentage products accounted for 3-53 % of the initial GSL. NITs were produced from DS-GSLs suggesting an alternative metabolism via desulfation for the food based GSLs. The metal ion dependency for NIT production for GNT and its DS form was investigated where it was shown that Fe(2+) increased NIT production, while Mg(2+) stimulated the formation of ITC.
Peruvian Maca (Lepidium peruvianum): (II) Phytochemical Profiles of Four Prime Maca Phenotypes Grown in Two Geographically-Distant Locations.[Pubmed:27127450]
Int J Biomed Sci. 2016 Mar;12(1):9-24.
Peruvian Maca crops (Lepidium peruvianum), grown in two geographically-distant cultivation sites located at similar altitudes in the highlands of the Peruvian Andes (Junin at 4,200 m a.s.l. and Ancash 4,150 m a.s.l.), were used in the study. Four prime Maca phenotypes, distinguished by hypocotyl colours labelled as "Yellow", "Purple", "Red" and "Black" were selected to determine distribution in levels and corresponding ratios between individual Glucosinolates (Glucotropaeolin and m-methylGlucotropaeolin) in an attempt to identify four Peruvian Maca phenotypes from analyses of powdered hypocotyls. There were highly significant differences (P<0.01) in hypocotyl weight/size of four Maca phenotypes harvested in two locations. The Junin crop represented a mostly "large" class (13.3 g) with "small" size hypocotyls (7.2 g), while a "small" class was predominant in Ancash (3.5 g). Powdered Yellow Maca showed significantly higher (P<0.001) microbial contamination than the other three, with Black Maca being the least infected. Only minor, statistically-confirmed differences were detected in nutritive characteristics between the four Maca phenotypes grown in Junin, however highly significant differences (P<0.01) in Glucosinolates existed between the Red and Black Maca grown in Junin and Ancash. Irrespective of the cultivation location, Red phenotypes showed the highest content of Total Glucosinolates, followed by Black and Purple, with the Yellow phenotype showing consistently lower levels. Highly significant P<0.01) differences determined in ratios of individual Glucosinolates between four Maca phenotypes grown in two locations, confirms an earlier assumption that sums of individual Glucosinolates, their ratios and profiles, may be feasible to explore in analytically identifying individual Maca phenotypes in pulverised marketed Maca products.
Benzyl Isothiocyanate Inhibits Prostate Cancer Development in the Transgenic Adenocarcinoma Mouse Prostate (TRAMP) Model, Which Is Associated with the Induction of Cell Cycle G1 Arrest.[Pubmed:26907265]
Int J Mol Sci. 2016 Feb 22;17(2):264.
Benzyl isothiocyanate (BITC) is a hydrolysis product of Glucotropaeolin, a compound found in cruciferous vegetables, and has been shown to have anti-tumor properties. In the present study, we investigated whether BITC inhibits the development of prostate cancer in the transgenic adenocarcinoma mouse prostate (TRAMP) mice. Five-week old, male TRAMP mice and their nontransgenic littermates were gavage-fed with 0, 5, or 10 mg/kg of BITC every day for 19 weeks. The weight of the genitourinary tract increased markedly in TRAMP mice and this increase was suppressed significantly by BITC feeding. H and E staining of the dorsolateral lobes of the prostate demonstrated that well-differentiated carcinoma (WDC) was a predominant feature in the TRAMP mice. The number of lobes with WDC was reduced by BITC feeding while that of lobes with prostatic intraepithelial neoplasia was increased. BITC feeding reduced the number of cells expressing Ki67 (a proliferation marker), cyclin A, cyclin D1, and cyclin-dependent kinase (CDK)2 in the prostatic tissue. In vitro cell culture results revealed that BITC decreased DNA synthesis, as well as CDK2 and CDK4 activity in TRAMP-C2 mouse prostate cancer cells. These results indicate that inhibition of cell cycle progression contributes to the inhibition of prostate cancer development in TRAMP mice treated with BITC.
Bioavailability and metabolism of benzyl glucosinolate in humans consuming Indian cress (Tropaeolum majus L.).[Pubmed:26610401]
Mol Nutr Food Res. 2016 Mar;60(3):652-60.
SCOPE: Benzyl isothiocyanate (BITC), which occurs in Brassicales, has demonstrated chemopreventive potency and cancer treatment properties in cell and animal studies. However, fate of BITC in human body is not comprehensively studied. Therefore, the present human intervention study investigates the metabolism of the glucosinolate (GSL) Glucotropaeolin and its corresponding BITC metabolites. Analyzing BITC metabolites in plasma and urine should reveal insights about resorption, metabolism, and excretion. METHODS AND RESULTS: Fifteen healthy men were randomly recruited for a cross-over study and consumed 10 g freeze-dried Indian cress as a liquid preparation containing 1000 mumol Glucotropaeolin. Blood and urine samples were taken at several time points and investigated by LC-ESI-MS/MS after sample preparation using SPE. Plasma contained high levels of BITC-glutathione (BITC-GSH), BITC-cysteinylglycine (BITC-CysGly), and BITC-N-acetyl-L-cysteine (BITC-NAC) 1-5 h after ingestion, with BITC-CysGly appearing as the main metabolite. Compared to human plasma, the main urinary metabolites were BITC-NAC and BITC-Cys, determined 4-6 h after ingestion. CONCLUSION: This study confirms that consumption of Indian cress increases the concentration of BITC metabolites in human plasma and urine. The outcome of this human intervention study supports clinical research dealing with GSL-containing innovative food products or pharmaceutical preparations.
Peruvian Maca (Lepidium peruvianum): (I) Phytochemical and Genetic Differences in Three Maca Phenotypes.[Pubmed:26508907]
Int J Biomed Sci. 2015 Sep;11(3):131-45.
Glucosinolates were previously reported as physiologically-important constituents present in Peruvian Maca (Lepidium peruvianum Chacon) and linked to various therapeutic functions of differently-colored Peruvian Maca hypocotyls. In two separate Trials, three colours of Maca hypocotyls "Black", "Red" and "Yellow" (termed "Maca phenotypes"), were selected from mixed crops of Peruvian Maca for laboratory studies as fresh and after being dried. Individual Maca phenotypes were cultivated in the highlands of the Peruvian Andes at 4,200m a.s.l. (Junin and Ninacaca). Glucosinolate levels, chromatographic HPLC profiles and DNA variability in the investigated Maca phenotypes are presented. Genotypic profiles were determined by the ISSR-PCR and RAPD techniques. Compared to the Black and Red phenotypes, the Yellow phenotype contained much lower Glucosinolate levels measured against Glucotropaeolin and m-methoxy-Glucotropaeolin standards, and exhibited different RAPD and ISSR-PCR reactions. The Red Maca phenotype showed the highest concentrations of Glucosinolates as compared to the Black and Yellow Maca. It appears that the traditional system used by natives of the Peruvian Andean highlands in preparing Maca as a vegetable dish (boiling dried Maca after soaking in water), to supplement their daily meals, is as effective as laboratory methods - for extracting Glucosinolates, which are considered to be one of the key bioactive constituents responsible for therapeutic functions of Peruvian Maca phenotypes. It is reasonable to assume that the HPLC and DNA techniques combined, or separately, may assist in determining ID and "Fingerprints" identifying individual Peruvian Maca phenotypes, hence confirming the authenticity of marketable Maca products. The above assumptions warrant further laboratory testing.
Methyl Jasmonate- and Light-Induced Glucosinolate and Anthocyanin Biosynthesis in Radish Seedlings.[Pubmed:26411013]
Nat Prod Commun. 2015 Jul;10(7):1211-4.
Radish sprouts and young seedlings are considered important dietary vegetables in Asian countries. In this study, we investigated the levels of glucosinolate and anthocyanin accumulation in radish seedlings in response to light and methyl jasmonate (MeJA) treatments. MeJA facilitated the accumulation of glucosinolate and anthocyanins under light conditions. The glucosinolate and anthocyanin contents in the radish seedlings that were exposed to light after MeJA treatment were higher than those of the seedlings that were grown in the dark without MeJA. At a concentration of 100 muM, MeJA led to the greatest accumulation of the most glucosinolates under both light and dark conditions. Under light conditions, the levels of glucoraphenin, glucoerucin, and Glucotropaeolin accumulation were 1.53-, 1.60-, and 1.30-fold higher, respectively, than those of the control. Remarkable accumulations of glucobrassicin were observed under light conditions (4.4-, 6.7-, and 7.8-fold higher than that of the control following the application of 100, 300, and 500 muM MeJA, respectively). The level of cyanidin in the 300 muM MeJA-treated seedlings was double of that in the control without MeJA treatment. The highest level of pelargonidin was observed after treatment with 500 muM MeJA under light conditions; this level was 1.73 times higher than that in the control. A similar trend of anthocyaninaccumulation was observed in the radish seedlings following MeJA treatment under dark conditions, but the levels of anthocyanins were considerably lower in the seedlings that were grown in the dark. Our findings suggest that light and low concentrations of MeJA enhance the accumulations of glucosinolates and anthocyanins during the development of radish seedlings.


