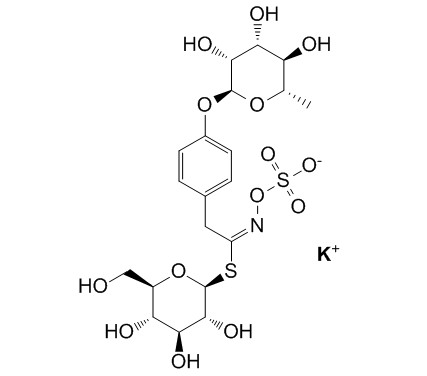
GlucomoringinCAS# 316165-49-8 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 316165-49-8 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | White powder |
| Formula | C20H28KNO14S2 | M.Wt | 609.7 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | 4-Rhamnosyloxybenzylglucosinolate potassium salt | ||
| Solubility | Soluble in methanol and water | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Glucomoringin Dilution Calculator

Glucomoringin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6402 mL | 8.2008 mL | 16.4015 mL | 32.803 mL | 41.0038 mL |
| 5 mM | 0.328 mL | 1.6402 mL | 3.2803 mL | 6.5606 mL | 8.2008 mL |
| 10 mM | 0.164 mL | 0.8201 mL | 1.6402 mL | 3.2803 mL | 4.1004 mL |
| 50 mM | 0.0328 mL | 0.164 mL | 0.328 mL | 0.6561 mL | 0.8201 mL |
| 100 mM | 0.0164 mL | 0.082 mL | 0.164 mL | 0.328 mL | 0.41 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Glucotropaeolin
Catalog No.:BCN8978
CAS No.:5115-71-9
- Glucoerucin
Catalog No.:BCN8977
CAS No.:15592-37-7
- Glucoberteroin
Catalog No.:BCN8976
CAS No.:245550-65-6
- Glucoiberin
Catalog No.:BCN8975
CAS No.:15592-34-4
- Glucobrassicanapin
Catalog No.:BCN8974
CAS No.:245550-58-7
- Sinalbin
Catalog No.:BCN8973
CAS No.:20196-67-2
- Glucocapparin
Catalog No.:BCN8972
CAS No.:15592-33-3
- Glucolimnanthin
Catalog No.:BCN8971
CAS No.:111810-95-8
- Glucobarbarin
Catalog No.:BCN8970
CAS No.:21087-78-5
- Phyllalbine
Catalog No.:BCN8969
CAS No.:4540-25-4
- Epiprogoitrin
Catalog No.:BCN8968
CAS No.:21087-74-1
- 4-Hydroxyglucobrassicin
Catalog No.:BCN8967
CAS No.:83327-20-2
- Lupinine
Catalog No.:BCN8981
CAS No.:486-70-4
- Glucoalyssin
Catalog No.:BCN8982
CAS No.:499-37-6
- Neoglucobrassicin potassium salt
Catalog No.:BCN8983
CAS No.:5187-84-8
- Gluconapin
Catalog No.:BCN8984
CAS No.:245550-57-6
- Progoitrin
Catalog No.:BCN8985
CAS No.:21087-77-4
- 18-Hydroxyspartioidine
Catalog No.:BCN8941
CAS No.:
- Heliotridine N-oxide
Catalog No.:BCN8944
CAS No.:
- Rinderine hydrochloride
Catalog No.:BCN8947
CAS No.:26131-12-4
- Jacoline N-oxide
Catalog No.:BCN8954
CAS No.:
- Merepoxine N-oxide
Catalog No.:BCN8956
CAS No.:
- 11-(Methylsulfinyl)undecylglucosinolate
Catalog No.:BCN8980
CAS No.:
- Pongamol
Catalog No.:BCN8986
CAS No.:484-33-3
In vitro anti-allergic activity of Moringa oleifera Lam. extracts and their isolated compounds.[Pubmed:31829185]
BMC Complement Altern Med. 2019 Dec 11;19(1):361.
BACKGROUND: Moringa oleifera Lam. is a commonly used plant in herbal medicine and has various reported bioactivities such as antioxidant, antimicrobial, anticancer and antidiabetes. It is rich in nutrients and polyphenols. The plant also has been traditionally used for alleviating allergic conditions. This study was aimed to examine the anti-allergic activity of M. oleifera extracts and its isolated compounds. METHOD: M. oleifera leaves, seeds and pods were extracted with 80% of ethanol. Individual compounds were isolated using a column chromatographic technique and elucidated based on the nuclear magnetic resonance (NMR) and electrospray ionisation mass spectrometry (ESIMS) spectral data. The anti-allergic activity of the extracts, isolated compounds and ketotifen fumarate as a positive control was evaluated using rat basophilic leukaemia (RBL-2H3) cells for early and late phases of allergic reactions. The early phase was determined based on the inhibition of beta-hexosaminidase and histamine release; while the late phase was based on the inhibition of interleukin (IL-4) and tumour necrosis factor (TNF-alpha) release. RESULTS: Two new compounds; ethyl-(E)-undec-6-enoate (1) and 3,5,6-trihydroxy-2-(2,3,4,5,6-pentahydroxyphenyl)-4H-chromen-4-one (2) together with six known compounds; quercetin (3), kaempferol (4), beta-sitosterol-3-O-glucoside (5), oleic acid (6), Glucomoringin (7), 2,3,4-trihydroxybenzaldehyde (8) and stigmasterol (9) were isolated from M. oleifera extracts. All extracts and the isolated compounds inhibited mast cell degranulation by inhibiting beta-hexosaminidase and histamine release, as well as the release of IL-4 and TNF-alpha at varying levels compared with ketotifen fumarate. CONCLUSION: The study suggested that M. oleifera and its isolated compounds potentially have an anti-allergic activity by inhibiting both early and late phases of allergic reactions.
Prospective role of mitochondrial apoptotic pathway in mediating GMG-ITC to reduce cytotoxicity in H2O2-induced oxidative stress in differentiated SH-SY5Y cells.[Pubmed:31541852]
Biomed Pharmacother. 2019 Nov;119:109445.
The antioxidant and neuroprotective activity of Glucomoringin isothiocyanate (GMG-ITC) have been reported in in vivo and in vitro models of neurodegenerative diseases. However, its neuroprotective role via mitochondrial-dependent pathway in a noxious environment remains unknown. The main objective of the present study was to unveil the mitochondrial apoptotic genes' profile and prospectively link with neuroprotective activity of GMG-ITC through its ROS scavenging. The results showed that pre-treatment of differentiated SH-SY5Y cells with 1.25mug/mL purified isolated GMG-ITC, significantly reduced reactive oxygen species (ROS) production level, compared to H2O2 control group, as evidenced by flow cytometry-based evaluation of ROS generation. Presence of GMG-ITC prior to development of oxidative stress condition, downregulated the expression of cyt-c, p53, Apaf-1, Bax, CASP3, CASP8 and CASP9 genes with concurrent upregulation of Bcl-2 gene in mitochondrial apoptotic signalling pathway. Protein Multiplex revealed significant decreased in cyt-c, p53, Apaf-1, Bax, CASP8 and CASP9 due to GMG-ITC pre-treatment in oxidative stress condition. The present findings speculated that pre-treatment with GMG-ITC may alleviate oxidative stress condition in neuronal cells by reducing ROS production level and protect the cells against apoptosis via neurodegenerative disease potential pathways.
Neuroprotective effects of glucomoringin-isothiocyanate against H2O2-Induced cytotoxicity in neuroblastoma (SH-SY5Y) cells.[Pubmed:31521693]
Neurotoxicology. 2019 Dec;75:89-104.
Neurodegenerative diseases (NDDs) are pathological conditions characterised by progressive damage of neuronal cells leading to eventual loss of structure and function of the cells. Due to implication of multi-systemic complexities of signalling pathways in NDDs, the causes and preventive mechanisms are not clearly delineated. The study was designed to investigate the potential signalling pathways involved in neuroprotective activities of purely isolated Glucomoringin isothiocyanate (GMG-ITC) against H2O2-induced cytotoxicity in neuroblastoma (SH-SY5Y) cells. GMG-ITC was isolated from Moringa oleifera seeds, and confirmed with NMR and LC-MS based methods. Gene expression analysis of phase II detoxifying markers revealed significant increase in the expression of all the genes involved, due to GMG-ITC pre-treatment. GMG-ITC also caused significant decreased in the expression of NF-kB, BACE1, APP and increased the expressions of IkB and MAPT tau genes in the differentiated cells as confirmed by multiplex genetic system analysis. The effect was reflected on the expressed proteins in the differentiated cells, where GMG-ITC caused increased in expression level of Nrf2, SOD-1, NQO1, p52 and c-Rel of nuclear factor erythroid factor 2 (Nrf2) and nuclear factor kappa-B (NF-kB) pathways respectively. The findings revealed the potential of GMG-ITC to abrogate oxidative stress-induced neurodegeneration through Nrf2 and NF-kB signalling pathways.
A Strategy to Deliver Precise Oral Doses of the Glucosinolates or Isothiocyanates from Moringa oleifera Leaves for Use in Clinical Studies.[Pubmed:31323988]
Nutrients. 2019 Jul 9;11(7). pii: nu11071547.
The tropical tree Moringa oleifera produces high yields of protein-rich leaf biomass, is widely used as a food source, contains an abundance of phytochemicals, and thus has great potential for chronic disease prevention and perhaps, treatment. We have developed and characterized standardized ways of preparing aqueous "teas" from moringa leaves to deliver precisely calibrated levels of phytochemicals for use in clinical trials. These phytochemicals, especially the glucosinolate Glucomoringin and the isothiocyanate moringin, produced from it following hydrolysis by the enzyme myrosinase, provide potent anti-inflammatory and cytoprotective indirect antioxidant activity. The taste of both hot and cold teas is palatable without the need for flavor masking. These teas can be easily and reproducibly prepared in underserved tropical regions of the world where moringa is cultivated. Isothiocyanate yield from a cold extraction was rapid and essentially complete after 30 min and its anti-inflammatory potential is comparable to that of equimolar purified moringin. A preparation similar to this may be safe to consume with respect to its bacterial titer even after 48 h without refrigeration. Thus, facile delivery of moringa tea to both adults and children for clinical evaluation of their effects on such conditions as autism, diabetes, and hypertension, is now possible.
Moringin from Moringa Oleifera Seeds Inhibits Growth, Arrests Cell-Cycle, and Induces Apoptosis of SH-SY5Y Human Neuroblastoma Cells through the Modulation of NF-kappaB and Apoptotic Related Factors.[Pubmed:31010127]
Int J Mol Sci. 2019 Apr 19;20(8). pii: ijms20081930.
In the last decades, glucosinolates (GLs), precursors of isothiocyanates (ITCs), have been studied mostly for their chemopreventive and chemotherapeutic properties. The aim of our research was to study the antiproliferative effect of 4-(alpha-L-rhamnopyranosyloxy) benzyl glucosinolate (Glucomoringin; GMG) bioactivated by myrosinase enzyme to form the corresponding isothiocyanate 4-(alpha-L-rhamnopyranosyloxy) benzyl C (moringin) in SH-SY5Y human neuroblastoma cells. We found that moringin significantly reduced SH-SY5Y cell growth in a time and concentration-dependent (p < 0.05, 0.01, and 0.001 vs. ctrl, after treatment with 16.4 microM moringin for 24, 48, and 72 h, respectively) manner through a mechanism involving the activation of apoptotic machinery. In addition, it altered the normal progression of cells through the cell cycle, increasing the cell population in both G2 and S phases, as well as decreasing that in the G1 phase. Studying the drug mechanism of action, we found that moringin was able to increase the expression of p53, p21, and Bax at both the protein and transcriptional level. Moreover, exposure of SH-SY5Y cells to moringin significantly increased the gene expression of both caspase 3 and 9 and enhanced their cleavage, thereby initiating an intrinsic apoptotic cascade. Finally, moringin inhibited nuclear translocation of NF-kappaB. Our study demonstrates the ability of moringin to reduce the growth of SH-SY5Y cells and reveals its mechanism of action, suggesting its promising role as an anticancer drug.
UPLC-Q-Orbitrap-MS(2) analysis of Moringa oleifera leaf extract and its antioxidant, antibacterial and anti-inflammatory activities.[Pubmed:30810361]
Nat Prod Res. 2019 Feb 27:1-5.
Moringa oleifera leaf acetone extract (MLE) was prepared. Phytochemicals of MLE and their antioxidant, antibacterial, and anti-inflammatory activities were evaluated. Results showed that MLE contained total phenolic content of 20.16 mg gallic acid equivalents/g dry weight. A total of 39 compounds were identified by mass spectrometry. The contents of acetyl-Glucomoringin, caffeoylquinic acid, feruloylquinic acid, and coumarylquinic acid were high. MLE had high DPPH. and ABTS(*+) scavenging activities and reducing powder. In addition, MLE could effectively inhibit S. aureus and B. subtilis, but little effect on E. coli was found. The anti-inflammatory effect of MLE was evaluated using a lipopolysaccharide (LPS) -induced RAW 264.7 cell model. MLE significantly inhibited nitric oxide (NO) production and inducible NO synthase (iNOS) mRNA levels in LPS-induced RAW 264.7 cells. The inhibitory activity increased in a dose-dependent manner. The bioactivities of MLE were related to its phenolic content and phenolic profiles.
A Combination of Moringin and Avenanthramide 2f Inhibits the Proliferation of Hep3B Liver Cancer Cells Inducing Intrinsic and Extrinsic Apoptosis.[Pubmed:30204484]
Nutr Cancer. 2018 Oct;70(7):1159-1165.
Moringin (MOR), a glycosyl-isothiocyanate obtained by myrosinase-catalyzed hydrolysis of the precursor 4-(alpha-l-rhamnosyloxy)-benzyl glucosinolate (Glucomoringin), found predominantly in the seeds of Moringa oleifera, shows anticancer effects against several cancer cell lines. Avenanthramide (AVN) 2f is a phytochemical purified from oats with antioxidant and anticancer properties. The aim of this study was to investigate the antiproliferative and proapoptotic effects of MOR and AVN 2f used alone and in combination on Hep3B cancer cells, which are highly resistant to conventional anticancer drugs. We found that a cocktail of MOR and AVN 2f significantly inhibited the Hep3B proliferation rate by markedly increasing the activity of caspases 2, 8, 9, and 3. Extrinsic apoptosis was induced by the AVN 2f-mediated activation of caspase 8, while the intrinsic apoptotic pathway was triggered by MOR-induced increase in the levels of intracellular reactive oxygen species, MOR-mediated activation of caspases 2 and 9 and the MOR-mediated downregulation of the prosurvival gene BIRC5. Our results suggest that the combination MOR + AVN 2f could be an effective chemopreventive cocktail against the development of hepatocarcinoma.
Nontoxic Glucomoringin-Isothiocyanate (GMG-ITC) Rich Soluble Extract Induces Apoptosis and Inhibits Proliferation of Human Prostate Adenocarcinoma Cells (PC-3).[Pubmed:30150582]
Nutrients. 2018 Aug 27;10(9). pii: nu10091174.
The incidence of prostate cancer malignancy along with other cancer types is increasing worldwide, resulting in high mortality rate due to lack of effective medications. Moringa oleifera has been used for the treatment of communicable and non-communicable ailments across tropical countries, yet, little has been documented regarding its effect on prostate cancer. We evaluated the acute toxicity and apoptosis inducing effect of Glucomoringin-isothiocyanate rich soluble extracts (GMG-ITC-RSE) from M. oleifera in vivo and in vitro, respectively. Glucomoringin was isolated, identified, and characterized using fundamental analytical chemistry tools where Sprague-Dawley (SD) rats, murine fibroblast (3T3), and human prostate adenocarcinoma cells (PC-3) were used for acute toxicity and bioassays experiments. GMG-ITC-RSE did not instigate adverse toxic reactions to the animals even at high doses (2000 mg/kg body weight) and affected none of the vital organs in the rats. The extract exhibited high levels of safety in 3T3 cells, where more than 90% of the cells appeared viable when treated with the extract in a time-dependent manner even at high dose (250 microg/mL). GMG-ITC-RSE significantly triggered morphological aberrations distinctive to apoptosis observed under microscope. These findings obviously revealed the putative safety of GMG-ITC-RSE in vivo and in vitro, in addition to its anti-proliferative effect on PC-3 cells.
A Combined Approach of NMR and Mass Spectrometry Techniques Applied to the alpha-Cyclodextrin/Moringin Complex for a Novel Bioactive Formulation (dagger).[Pubmed:30011859]
Molecules. 2018 Jul 13;23(7). pii: molecules23071714.
Moringin, obtained via enzymatic conversion of the glucosinolate precursor Glucomoringin, is an uncommon member of the isothiocyanate class, and has been proven to possess a broad range of biological activities such as antitumor activity, protection against neurodegenerative disorders and bactericidal effects. Since moringin is weakly soluble in water and unstable in aqueous medium, cyclodextrins (CDs) were considered for the development of a new moringin formulation, with a view to improving its solubility and stability in aqueous solution for use as an anti-inflammatory. A combined structural study using proton nuclear magnetic resonance ((1)H-NMR), diffusion-ordered spectroscopy (DOSY) and ion mobility mass spectrometry (IM-MS) is reported, highlighting the formation of a 1:1 alpha-CD/moringin inclusion complex. The association constant K was determined (1300 M(-1) at 300 K). Completion of the structural characterization was performed by T-ROESY and MS/MS experiments, which evidenced the mode of penetration of moringin into alpha-CD. Finally, the "chaperone-like" properties of alpha-CD with respect to the stability of moringin have been highlighted.
Bifunctional mannoside-glucosinolate glycoconjugates as enzymatically triggered isothiocyanates and FimH ligands.[Pubmed:29938295]
Org Biomol Chem. 2018 Jul 4;16(26):4900-4913.
Glucosinolates are sulfur-containing secondary metabolites found in plants of the Brassicale order. They are precursors of isothiocyanate species, resulting from C-S hydrolysis catalysed by the thioglucohydrolase myrosinase. We describe the synthesis of bifunctional glucosinolate-mannoside glycoconjugates combining both the structural features of a substrate of myrosinase and a ligand of the lectin FimH. We show that these glycoconjugates serve as enzyme substrates and that myrosinase can indeed hydrolyze the glucosinolate moiety with affinities (KM, Vmax) comparable to the natural substrates Glucomoringin and sinigrin. This enzymatic hydrolysis of the thioglycosidic bond led to the efficient formation of an isothiocyanate which was assessed by the formation of the corresponding dithiocarbamate derivatives. Finally, we show that our synthetic bifunctional glycoconjugates also serve as FimH ligands where the glucosinolate moiety does not hamper the interaction with the lectin. Our findings set the stage for an original bioconjugation tool, allowing for myrosinase-triggered specific labelling of lectins using glucosinolate glycoconjugates as non-toxic, water soluble isothiocyanate precursors.
Wild and domesticated Moringa oleifera differ in taste, glucosinolate composition, and antioxidant potential, but not myrosinase activity or protein content.[Pubmed:29789671]
Sci Rep. 2018 May 22;8(1):7995.
Taste drives consumption of foods. The tropical tree Moringa oleifera is grown worldwide as a protein-rich leafy vegetable and for the medicinal value of its phytochemicals, in particular its glucosinolates, which can lead to a pronounced harsh taste. All studies to date have examined only cultivated, domestic variants, meaning that potentially useful variation in wild type plants has been overlooked. We examine whether domesticated and wild type M. oleifera differ in myrosinase or glucosinolate levels, and whether these different levels impact taste in ways that could affect consumption. We assessed taste and measured levels of protein, glucosinolate, myrosinase content, and direct antioxidant activity of the leaves of 36 M. oleifera accessions grown in a common garden. Taste tests readily highlighted differences between wild type and domesticated M. oleifera. There were differences in direct antioxidant potential, but not in myrosinase activity or protein quantity. However, these two populations were readily separated based solely upon their proportions of the two predominant glucosinolates (Glucomoringin and glucosoonjnain). This study demonstrates substantial variation in glucosinolate composition within M. oleifera. The domestication of M. oleifera appears to have involved increases in levels of Glucomoringin and substantial reduction of glucosoonjnain, with marked changes in taste.
Isothiocyanate from Moringa oleifera seeds mitigates hydrogen peroxide-induced cytotoxicity and preserved morphological features of human neuronal cells.[Pubmed:29723199]
PLoS One. 2018 May 3;13(5):e0196403.
Reactive oxygen species are well known for induction of oxidative stress conditions through oxidation of vital biomarkers leading to cellular death via apoptosis and other process, thereby causing devastative effects on the host organs. This effect is believed to be linked with pathological alterations seen in several neurodegenerative disease conditions. Many phytochemical compounds proved to have robust antioxidant activities that deterred cells against cytotoxic stress environment, thus protect apoptotic cell death. In view of that we studied the potential of Glucomoringin-isothiocyanate (GMG-ITC) or moringin to mitigate the process that lead to neurodegeneration in various ways. Neuroprotective effect of GMG-ITC was performed on retinoic acid (RA) induced differentiated neuroblastoma cells (SHSY5Y) via cell viability assay, flow cytometry analysis and fluorescence microscopy by means of acridine orange and propidium iodide double staining, to evaluate the anti-apoptotic activity and morphology conservation ability of the compound. Additionally, neurite surface integrity and ultrastructural analysis were carried out by means of scanning and transmission electron microscopy to assess the orientation of surface and internal features of the treated neuronal cells. GMG-ITC pre-treated neuron cells showed significant resistance to H2O2-induced apoptotic cell death, revealing high level of protection by the compound. Increase of intracellular oxidative stress induced by H2O2 was mitigated by GMG-ITC. Thus, pre-treatment with the compound conferred significant protection to cytoskeleton and cytoplasmic inclusion coupled with conservation of surface morphological features and general integrity of neuronal cells. Therefore, the collective findings in the presence study indicated the potentials of GMG-ITC to protect the integrity of neuron cells against induced oxidative-stress related cytotoxic processes, the hallmark of neurodegenerative diseases.
Identification of Glucosinolates in Seeds of Three Brassicaceae Species Known to Hyperaccumulate Heavy Metals.[Pubmed:27981800]
Chem Biodivers. 2017 Mar;14(3).
Plants from the Brassicaceae family are known to contain secondary metabolites called glucosinolates. Our goal was to establish by LC/MS the glucosinolate profile of seeds of three Brassicaceae species known to hyperaccumulate heavy metals. We investigated Alyssum fallacinum auct. non Hausskn., Iberis intermedia Guers., and Noccaea caerulescens (J. Presl & C. Presl) F. K. Mey. Our results indicate that A. fallacinum seeds contain glucoiberin and glucoibervirin, which had not been previously identified in this plant. Furthermore, we report for the first time the presence of glucoiberin, glucoibervirin, glucotropaeolin, and sinigrin in I. intermedia. We have detected for the first time glucoconringiin in N. caerulescens. In addition, glucosinalbin, 4-hydroxyglucobrassicin, and Glucomoringin were also detected.
Moringin activates Wnt canonical pathway by inhibiting GSK3beta in a mouse model of experimental autoimmune encephalomyelitis.[Pubmed:27784989]
Drug Des Devel Ther. 2016 Oct 4;10:3291-3304.
Aberrant canonical Wnt-beta-catenin signaling has been reported in multiple sclerosis (MS), although the results are controversial. The present study aimed to examine the role of the Wnt-beta-catenin pathway in experimental MS and also to test moringin (4-[alpha-L-rhamnopyranosyloxy]-benzyl isothiocyanate), resulting from exogenous myrosinase hydrolysis of the natural phytochemical Glucomoringin 4(alpha-L-rhamnosyloxy)-benzyl glucosinolate as a modulator of neuroinflammation via the beta-catenin-PPARgamma axis. Experimental autoimmune encephalomyelitis (EAE), the most common model of MS, was induced in C57BL/6 mice by immunization with MOG35-55. Released moringin (10 mg/kg Glucomoringin +5 muL myrosinase/mouse) was administered daily for 1 week before EAE induction and continued until mice were killed on day 28 after EAE induction. Our results clearly showed that the Wnt-beta-catenin pathway was downregulated in the EAE model, whereas moringin pretreatment was able to avert this. Moringin pretreatment normalizes the aberrant Wnt-beta-catenin pathway, resulting in GSK3beta inhibition and beta-catenin upregulation, which regulates T-cell activation (CD4 and FoxP3), suppresses the main inflammatory mediators (IL-1beta, IL-6, and COX2), through activation of PPARgamma. In addition, moringin attenuates apoptosis by reducing the expression of the Fas ligand and cleaved caspase 9, and in parallel increases antioxidant Nrf2 expression in EAE mice. Taken together, our results provide an interesting discovery in identifying moringin as a modulator of the Wnt-beta-catenin signaling cascade and as a new potential therapeutic target for MS treatment.
Moringa oleifera Lam: Targeting Chemoprevention.[Pubmed:27644601]
Asian Pac J Cancer Prev. 2016;17(8):3675-86.
Moringa oleifera Lam, family Moringaceae, is a perennial plant which is called various names, but is locally known in Malaysia as "murungai" or "kelor". Glucomoringin, a glucosinolate with from M. oleifera is a major secondary metabolite compound. The seeds and leaves of the plant are reported to have the highest amount of glucosinolates. M. oleifera is well known for its many uses health and benefits. It is claimed to have nutritional, medicinal and chemopreventive potentials. Chemopreventive effects of M. oleifera are expected due to the existence of glucosinolate which it is reported to have the ability to induce apoptosis in anticancer studies. Furthermore, chemopreventive value of M. oleifera has been demonstrated in studies utilizing its leaf extract to inhibit the growth of human cancer cell lines. This review highlights the advantages of M. oleifera targeting chemoprevention where glucosinolates could help to slow the process of carcinogenesis through several molecular targets. It is also includes inhibition of carcinogen activation and induction of carcinogen detoxification, anti-inflammatory, anti-tumor cell proliferation, induction of apoptosis and inhibition of tumor angiogenesis. Finally, for synergistic effects of M. oleifera with other drugs and safety, essential for chemoprevention, it is important that it safe to be consumed by human body and works well. Although there is promising evidence about M. oleifera in chemoprevention, extensive research needs to be done due to the expected rise of cancer in coming years and to gain more information about the mechanisms involved in M. oleifera influence, which could be a good source to inhibit several major mechanisms involved in cancer development.


