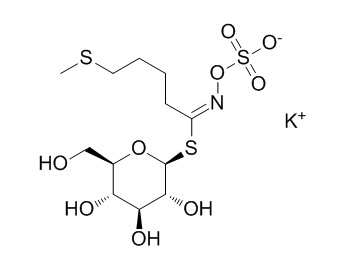
GlucoerucinCAS# 15592-37-7 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 15592-37-7 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Beige powder |
| Formula | C12H22KNO9S3 | M.Wt | 459.6 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | 4-Methylthiobutylglucosinolate potassium salt | ||
| Solubility | Soluble in methanol and water | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Glucoerucin Dilution Calculator

Glucoerucin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1758 mL | 10.879 mL | 21.7581 mL | 43.5161 mL | 54.3951 mL |
| 5 mM | 0.4352 mL | 2.1758 mL | 4.3516 mL | 8.7032 mL | 10.879 mL |
| 10 mM | 0.2176 mL | 1.0879 mL | 2.1758 mL | 4.3516 mL | 5.4395 mL |
| 50 mM | 0.0435 mL | 0.2176 mL | 0.4352 mL | 0.8703 mL | 1.0879 mL |
| 100 mM | 0.0218 mL | 0.1088 mL | 0.2176 mL | 0.4352 mL | 0.544 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Glucoberteroin
Catalog No.:BCN8976
CAS No.:245550-65-6
- Glucoiberin
Catalog No.:BCN8975
CAS No.:15592-34-4
- Glucobrassicanapin
Catalog No.:BCN8974
CAS No.:245550-58-7
- Sinalbin
Catalog No.:BCN8973
CAS No.:20196-67-2
- Glucocapparin
Catalog No.:BCN8972
CAS No.:15592-33-3
- Glucolimnanthin
Catalog No.:BCN8971
CAS No.:111810-95-8
- Glucobarbarin
Catalog No.:BCN8970
CAS No.:21087-78-5
- Phyllalbine
Catalog No.:BCN8969
CAS No.:4540-25-4
- Epiprogoitrin
Catalog No.:BCN8968
CAS No.:21087-74-1
- 4-Hydroxyglucobrassicin
Catalog No.:BCN8967
CAS No.:83327-20-2
- 4-Methoxyglucobrassicin
Catalog No.:BCN8966
CAS No.:83327-21-3
- Glucoarabin
Catalog No.:BCN8965
CAS No.:67920-64-3
- Glucotropaeolin
Catalog No.:BCN8978
CAS No.:5115-71-9
- Glucomoringin
Catalog No.:BCN8979
CAS No.:316165-49-8
- Lupinine
Catalog No.:BCN8981
CAS No.:486-70-4
- Glucoalyssin
Catalog No.:BCN8982
CAS No.:499-37-6
- Neoglucobrassicin potassium salt
Catalog No.:BCN8983
CAS No.:5187-84-8
- Gluconapin
Catalog No.:BCN8984
CAS No.:245550-57-6
- Progoitrin
Catalog No.:BCN8985
CAS No.:21087-77-4
- 18-Hydroxyspartioidine
Catalog No.:BCN8941
CAS No.:
- Heliotridine N-oxide
Catalog No.:BCN8944
CAS No.:
- Rinderine hydrochloride
Catalog No.:BCN8947
CAS No.:26131-12-4
- Jacoline N-oxide
Catalog No.:BCN8954
CAS No.:
- Merepoxine N-oxide
Catalog No.:BCN8956
CAS No.:
A colorimetric sensor array for the discrimination of glucosinolates.[Pubmed:32480264]
Food Chem. 2020 May 26;328:127149.
A novel approach for the discrimination of different glucosinolates (sinigrin, progoitrin, gluconapin, 4-methoxyglucobrassicin, glucoraphanin, glucobrassicin, glucoiberin, glucobrassicanapin, glucoraphenin, and Glucoerucin) using a colorimetric sensor array (CSA) is reported herein. The developed CSA technique exhibited an acceptable linearity (r(2) >/= 0.97) over a concentration range of 0-150 muM for the 10 glucosinolates. The CSA coupled with principal component analysis and hierarchical cluster analysis correctly distinguished the majority of glucosinolate samples according to their type. In addition, the CSA coupled with linear discriminant analysis correctly classified the majority of 8 kinds of cruciferous vegetable samples with an overall accuracy of 94%. Furthermore, the partial least squares regression results showed that the CSA responses were correlated with the concentration in a correlation coefficient (Rp) range of 0.813-0.964. These results demonstrate that the described procedure based on the CSA technique could be useful for the rapid discrimination of different glucosinolates.
Profiling of Individual Desulfo-Glucosinolate Content in Cabbage Head (Brassica oleracea var. capitata) Germplasm.[Pubmed:32316621]
Molecules. 2020 Apr 17;25(8). pii: molecules25081860.
Individual glucosinolates (GSLs) were assessed to select cabbage genotypes for a potential breeding program. One hundred forty-six cabbage genotypes from different origins were grown in an open field from March to June 2019; the cabbage heads were used for GSL analyses. Seven aliphatics [glucoiberin (GIB), progoitrin (PRO), epi-progoitrin (EPI), sinigrin (SIN), glucoraphanin (GRA), Glucoerucin (GER) and gluconapin (GNA)], one aromatic [gluconasturtiin (GNS)] and four indolyl GSLs [glucobrassicin (GBS), 4-hydroxyglucobrassicin (4HGBS), 4-methoxyglucobrassicin (4MGBS), neoglucobrassicin (NGBS)] were found this study. Significant variation was observed in the individual GSL content and in each class of GSLs among the cabbage genotypes. Aliphatic GSLs were predominant (58.5%) among the total GSLs, followed by indolyl GSL (40.7%) and aromatic GSLs (0.8%), showing 46.4, 51.2 and 137.8% coefficients of variation, respectively. GIB, GBS and NGBS were the most common GSLs found in all genotypes. GBS was the most dominant GSL, with an average value of 3.91 micromol g(-1) (0.79 to 13.14 micromol g(-1)). SIN, GIB, PRO and GRA were the other major GSLs, showing average values of 3.45, 1.50, 0.77 and 0.62 micromol g(-1), respectively. The genotypes with relatively high contents of GBS, SIN, GIB and GRA warrant detailed studies for future breeding programs since the hydrolysis products of these GSLs have several anti-cancer properties.
Evolution of important glucosinolates in three common Brassica vegetables during their processing into vegetable powder and in vitro gastric digestion.[Pubmed:31915766]
Food Funct. 2020 Jan 29;11(1):211-220.
Evolution of important glucosinolates (GLSs), namely, sinigrin, glucoraphanin, Glucoerucin and glucobrassicin, in three commonly consumed Brassica vegetables viz. white cabbage, Chinese cabbage and bok choy during their processing into vegetable powder was investigated. Drying was noted to be a major processing step causing significant losses of GLSs. Interestingly, different GLSs and even the same GLSs in different vegetables showed different thermal stabilities during drying. The stability of GLSs in vegetable powder during in vitro gastric digestion was also studied. Glucoraphanin exhibited the highest stability while glucobrassicin was the most vulnerable GLS under in vitro gastric conditions. White cabbage is found to be a promising material for the production of vegetable powder as it contains high contents of GLSs, especially glucoraphanin and Glucoerucin, which are important precursors of anticarcinogenic compounds, namely sulforaphane and erucin. These two GLSs were also noted to be stable during in vitro gastric digestion.
Effect of pretreatment on bioactive compounds in wild rocket juice.[Pubmed:31749470]
J Food Sci Technol. 2019 Dec;56(12):5234-5242.
The aim of the study was to determine the effect of pretreatment with hot water or steaming on glucosinolates, polyphenols contents and antioxidant capacity in obtained raw juices. Moreover, in vitro cytotoxic activity of the raw juice to the cells derived from the gastrointestinal tract, including the small intestine (IEC-6 cell line), colon (Caco-2 cell line) and the liver (HepG2 cell line) were also investigated. The dominant glucosinolates in the wild rocket leaves were glucoraphanin (36%) and dimeric 4-mercaptobutyl (30%), followed by glucosativin and Glucoerucin, 11% per each. Glucothiobeinin (6%), glucobrassicin (1%), 4-methoxyglucobrassicin (1%) and two unidentified compounds (4%) were also detected in rocket leaves. In terms of phenolic compounds, quercetin constituted the majority (55%) and the rest composed of hydroxycinnamic acids. In raw juices produced from steamed, pretreatment with hot water and untreated (control) leaves, glucosinolate contents were lower about 21%, 37% and 53%, respectively, than their levels in the raw material. The highest content of polyphenols among the juices tested (45.4 mg/100 g fresh weight) and antioxidant capacity (5.8 micromol Trolox/1 g f.w.) was recorded in the raw juice from pretreated leaves with hot water. The wild rocket raw juice concentrations responsible for a 50% reduction in Caco-2 and HepG2 cell viability were estimated at 1.87 +/- 0.08 mg/mL and 3.54 +/- 0.29 mg/mL. The viability of the IEC-6 cells was reduced by only 19.04%, at the maximum concentration (3.6 mg/mL) of the raw juice.
Natural Variation of Glucosinolates and Their Breakdown Products in Broccoli (Brassica oleracea var. italica) Seeds.[Pubmed:31631662]
J Agric Food Chem. 2019 Nov 13;67(45):12528-12537.
Seeds of 32 pure lines and 6 commercial broccoli cultivars were used to investigate variation in glucosinolates and their breakdown products. The aliphatic glucosinolate content was 54.5-218.7 mumol/g fresh weight, accounting for >90% of the total glucosinolates. The major glucosinolates found were glucoraphanin and Glucoerucin in 27 samples and progoitrin in 7 samples. A gas chromatography-flame ionization detector (GC-FID) method was used to identify glucosinolate breakdown products; nine products were directly determined using standards. Using Arabidopsis thaliana lines myb28myb29 and Landsberg erecta to hydrolyze each reference glucosinolate, seven products were tentatively identified. 4-(Methylsulfinyl)butyl isothiocyanate and 5-(methylsulfinyl)pentanenitrile contents were 2.6-91.1 mumol/g fresh weight and 0-35.4 mumol/g fresh weight, respectively, with epithionitriles being more common than nitriles in accessions rich in alkenyl glucosinolate. Additionally, (S)-5-vinyl-1,3-oxazolidine-2-thione was detected in accessions rich in progoitrin. Specific lines with altered glucosinolate profiles and breakdown products were obtained and discussed according to the putative glucosinolate metabolism pathway.
Eruca sativa Meal against Diabetic Neuropathic Pain: An H2S-Mediated Effect of Glucoerucin.[Pubmed:31430978]
Molecules. 2019 Aug 19;24(16). pii: molecules24163006.
: The management of pain in patients affected by diabetic neuropathy still represents an unmet therapeutic need. Recent data highlighted the pain-relieving efficacy of glucosinolates deriving from Brassicaceae. The purpose of this study was to evaluate the anti-hyperalgesic efficacy of Eruca sativa defatted seed meal, along with its main glucosinolate, Glucoerucin (GER), on diabetic neuropathic pain induced in mice by streptozotocin (STZ). The mechanism of action was also investigated. Hypersensitivity was assessed by paw pressure and cold plate tests after the acute administration of the compounds. Once bio-activated by myrosinase, both E. sativa defatted meal (1 g kg(-)(1) p.o.) and GER (100 micromol kg(-)(1) p.o., equimolar to meal content) showed a dose-dependent pain-relieving effect in STZ-diabetic mice, but the meal was more effective than the glucosinolate. The co-administration with H2S scavengers abolished the pain relief mediated by both E. sativa meal and GER. Their effect was also prevented by selectively blocking Kv7 potassium channels. Repeated treatments with E. sativa meal did not induce tolerance to the anti-hypersensitive effect. In conclusion, E. sativa meal can be suggested as a new nutraceutical tool for pain relief in patients with diabetic neuropathy.
Assessing the Fate and Bioavailability of Glucosinolates in Kale (Brassica oleracea) Using Simulated Human Digestion and Caco-2 Cell Uptake Models.[Pubmed:31374175]
J Agric Food Chem. 2019 Aug 28;67(34):9492-9500.
Glucosinolates and their hydrolysis products were characterized in fresh and in in vitro gastric and intestinal digesta of Dinosaur kale (Brassica oleracea L var. palmifolia DC). In fresh kale, glucoraphanin, sinigrin, gluconapin, gluconasturtiin, Glucoerucin, glucobrasscin, and 4-methoxylglucobrassicin were identified. After 120 min of gastric digestion, the levels of glucoraphanin, sinigrin, and gluconapin decreased, and no Glucoerucin or glucobrasscin was detected. However, a concomitant increase in the glucosinolate hydrolysis products allyl nitrile, 3-butenyl isothiocyanate, phenylacetonitrile, and sulforaphane was observed. This trend continued through intestinal digestion. After 120 min, the levels of allyl nitrile, 3-butenyl isothiocyanate, phenylacetonitrile, and sulforaphane were 88.19 +/- 5.85, 222.15 +/- 30.26, 129.17 +/- 17.57, and 13.71 +/- 0.62 pmol/g fresh weight, respectively. Intestinal digesta were then applied to Caco-2 cell monolayers to assess the bioavailability. After 6 h of incubation, no glucosinolates were detected and the percentage of total cellular uptake of the glucosinolate hydrolysis products ranged from 29.35% (sulforaphane) to 46.60% (allyl nitrile).
Bunias erucago L.: Glucosinolate Profile and In Vitro Biological Potential.[Pubmed:30791395]
Molecules. 2019 Feb 19;24(4). pii: molecules24040741.
Bunias erucago belongs to the Brassicaceae family, which represents a forgotten crop of the Euro-Mediterranean area. The aim of the present study was to determine the glucosinolate profile in different plant parts and biological properties (antioxidant, anticholinesterase, and cytotoxic activities) of the isolates containing glucosinolate breakdown products. The chemical profiles were determined by using HPLC-PDA-MS/MS of desulfoglucosinolates and GC-MS of glucosinolate degradation products. The analysis of B. erucago showed the presence of seven glucosinolates: gluconapin (1), glucoraphasatin (2), glucoraphenin (3), Glucoerucin (4), glucoraphanin (5), glucotropaeolin (6), and glucosinalbin (7). The total glucosinolate content ranged from 7.0 to 14.6 micromol/g of dry weight, with the major glucosinolate glucosinalbin in all parts. The antioxidant activity of all volatile isolates was not notable. At a tested concentration of 227 mug/mL, flower hydro-distillate (FH) showed good AChE inhibition, i.e., 40.9%, while root hydro-distillate (RH) had good activity against BChE, i.e., 54.3%. FH showed the best activity against both tested human bladder cancer cell lines, i.e., against T24 after 72 h, which have IC50 of 16.0 mug/mL, and against TCCSUP after 48 h with IC50 of 7.8 mug/mL, and can be considered as highly active. On the other hand, RH showed weak activity against tested cancer cells.
Simultaneous direct determination of 15 glucosinolates in eight Brassica species by UHPLC-Q-Orbitrap-MS.[Pubmed:30711096]
Food Chem. 2019 Jun 1;282:127-133.
Glucosinolates (GLS) have been reported to have significant anti-oxidative, antimicrobial, and anti-cancer activities. The current study was aimed to develop an analytical method for glucosinolate quantitation in eight Brassica species from Gwangju, Republic of Korea. For this purpose the UHPLC-Q-Orbitrap-MS technique was used and validated for optimal extraction conditions, detection and quantitation limits, linearity, precision, and accuracy. According to the results of GLS profiling, the total GLS concentration decreased in the order of cabbage>broccoli>cauliflower>mustard>kimchi cabbage>young radish approximately kale. All Brassica species contained Glucoerucin (GER) and glucobrassicin (GBR) as major GLS with the high levels in cabbage (5.913muM/g) and broccoli (1.723muM/g), respectively. The contents of minor GLS were species-dependent, and could therefore be used for Brassica species classification.
Anticancer properties of erucin, an H2 S-releasing isothiocyanate, on human pancreatic adenocarcinoma cells (AsPC-1).[Pubmed:30632211]
Phytother Res. 2019 Mar;33(3):845-855.
Plants of the Brassicaceae family are well-known for containing the glucosinolate myrosinase system, which is able to release isothiocyanates after plant biotic and abiotic lesions. Erucin (ERU; 1-isothiocyanato-4-(methylthio)-butane), an isothiocyanate particularly abundant in arugula (Eruca sativa Mill., Eruca vesicaria L., etc.), derives from the hydrolysis of the glucosinolate Glucoerucin by the enzyme myrosinase. Many other natural isothiocyanates influence cancer cells and, in particular, induce antiproliferative effects at relatively high concentrations. Similar antiproliferative effects have also been shown by the newly emerging gasotransmitter hydrogen sulfide (H2 S) and by H2 S-releasing compounds. In a previous study, our group demonstrated that isothiocyanates release H2 S in biological environments. In this work, we demonstrated the H2 S-donor properties of ERU in pancreatic adenocarcinoma cells (AsPC-1) and delineated its profile as a chemopreventive or anticancer agent. Indeed, ERU showed significant antiproliferative effects: ERU inhibited AsPC-1 cell viability at relatively high concentrations (30-100 muM). Moreover, ERU inhibited cell migration, altered the AsPC-1 cell cycle, and exhibited proapoptotic effects. Finally, ERU inhibited ERK1/2 phosphorylation. This mechanism is particularly important in AsPC-1 cells because they are characterized by a mutation in KRAS that determines KRAS hyperactivation followed by MAP-kinase hyperphosphorylation, which plays a pivotal role in pancreatic cancer proliferation, growth, and survival.
Combined effect of ultrasound treatment and exogenous phytohormones on the accumulation of bioactive compounds in broccoli florets.[Pubmed:30274889]
Ultrason Sonochem. 2019 Jan;50:289-301.
Postharvest treatments such as wounding, ultrasound (US) and the exogenous application of ethylene (ET) and methyl jasmonate (MJ) have been studied as an effective tool to improve the content of secondary metabolites in fresh produce. The present study evaluated the immediate and late response (storage for 72h at 15 degrees C) to US treatment (20min, frequency 24kHz, amplitude 100mum) alone and combined with exogenous MJ (250ppm) and/or ET (1000ppm) on glucosinolates, isothiocyanates, phenolic compounds and ascorbic acid content in broccoli florets. US treatment increased the extractability of glucosinolates [glucoraphanin (795%), 4-hydroxy glucobrassicin (153%), glucobrassicin (78.6%)] and phenolics [1-sinapoyl-2-feruloylgentiobiose (57.23%)] as compared with the control (CT). The combined application of MJ and US in broccoli florets, induced a synergistic effect on the accumulation of 4-hydroxy glucobrassicin (187.1%), Glucoerucin (111.92%), gluconasturtiin (755.9%), neoglucobrassicin (232.8%), 3-O-caffeoylquinic acid (73.4%), 1-sinapoyl-2-ferulolylgentiobiose (56.0%), and 1,2,2-trisinapoylgentiobiose (136.7%) at 72h of storage. Interestingly, when the three stressors were applied together the synergistic effect of US+MJ observed on the accumulation of glucosinolates and phenolics was repressed. In general, the ascorbic acid content was not affected by US treatment and decreased in most samples during storage. However, when MJ+ET were applied, the content of total ascorbic acid was significantly reduced in CT+MJ+ET and US+MJ+ET samples after 72h of storage by 53.4% and 86.6%, respectively, as compared with CT 0h samples. Based on the results herein obtained, the application of US can be an effective tool to enhance the extractability of certain glucocosinolate and phenolic compounds in broccoli. Moreover, due to the synergistic effect observed on the accumulation of bioactive compounds, the combined application of US and MJ could be a practical approach to yield higher levels of glucosinolates and phenolic compounds in broccoli during storage.
Influence of different light conditions and time of sprouting on harmful and beneficial aspects of rutabaga sprouts in comparison to their roots and seeds.[Pubmed:29876936]
J Sci Food Agric. 2019 Jan 15;99(1):302-308.
BACKGROUND: This study aimed to evaluate the presence and content of selected phytochemicals, namely glucosinolates, fatty acids and phenolic compounds, in rutabaga (Brassica napus L. var. napobrassica) sprouts grown under various light conditions, in comparison to rutabaga seeds and roots. As rutabaga sprouts are likely to become new functional food, special emphasis was placed on the related risks of progoitrin and erucic acid presence - compounds with proven antinutritive properties. RESULTS: Time of sprouting significantly decreased progoitrin content, especially after 10 days (by 91.5%) and 12 days (by 97.5%), as compared to 8 days. In addition, sprouts grown under dark conditions showed 27%, 60% and 17% reduction in progoitrin level in 8, 10 and 12 days after sowing, respectively, as compared to sprouts grown under natural conditions. Progoitrin was found to be the predominant glucosinolate in rutabaga seeds (804.07 +/- 60.89 mg 100 g(-1) dry weight (DW)), accompanied by Glucoerucin (157.82 +/- 21.04 mg 100 g(-1) DW), also found in the roots (82.20 +/- 16.53 mg 100 g(-1) DW). Among the unsaturated fatty acids in rutabaga sprouts, erucic, linoleic, linolenic and gondoic acids decreased significantly, and only oleic acid increased as germination days progressed. The amount of harmful erucic acid in rutabaga sprouts was found to vary between 1.8% and 7%, depending on the day of seeding or light conditions, as compared to 42.5% in the seeds. CONCLUSION: The evaluated rutabaga products showed a wide content range of potentially antinutritive compounds, sprouts having the lowest amounts of erucic acid and progoitrin. (c) 2018 Society of Chemical Industry.
Anti-fibrotic potential of a Matthiola arabica isothiocyanates rich fraction: impact on oxidative stress, inflammatory and fibrosis markers.[Pubmed:29441888]
Pharmazie. 2017 Oct 1;72(10):614-624.
The present study is the first one to investigate the glucosinolates (GLS) profile and anti-fibrotic effect of isothiocyanates (ITCs) rich fraction of Matthiola arabica (Brassicaceae) using an experimental model of liver fibrosis in rats. Five GLS (ethyl glucosinolate, gluconapin, glucodehydroerucin, Glucoerucin and glucoraphanin) were identified by gas liquid chromatography-mass spectrometric (GLC-MS) analysis of their hydrolysis products, produced by the natural autolysis and exogenous myrosinase hydrolysis using one and two units of the enzyme. Spectrophotometric determination of the total intact GLS revealed that content in the fresh sample was 1.8 times higher than in the dry one. ITCs rich fraction was prepared by natural autolysis of the fresh aerial part. Male albino rats were given carbon tetrachloride (CCl4) (0.5 ml/kg, twice a week) and/or ITCs -rich fraction (30 mg/ kg, three times a week) for six weeks. Liver function, different oxidative stress, inflammatory and fibrosis markers were investigated. Treatment of animals with ITCs rich fraction significantly counteracted the changes in liver function induced by CCl4. Histopathological examination under both light and electron microscope showed the anti-fibrotic effect of ITCs rich fraction. This finding was confirmed with the markedly improved liver fibrosis markers with ITCs rich fraction co-treatment. In elucidation of anti-fibrotic mechanisms of ITCs rich fraction, the significant glutathione depletion and lipid peroxidation caused by CCl4 intoxication was restored by ITCs rich fraction co-treatment. Besides, ITCs rich fraction showed an anti-inflammatory effect through its ability to counteract the significant increase in nuclear factor kappa B (NF-kappaB), cyclooxygenase-2 (COX-2) expression, tumor necrosis factor alpha (TNF-alpha) and interleukin-6 (IL-6) levels in liver tissue that caused by CCl4 intoxication. These findings indicate that ITCs-rich fraction of M. arabica possesses a promising anti-fibrotic effect which can be attributed to its antioxidant and anti-inflammatory properties.


