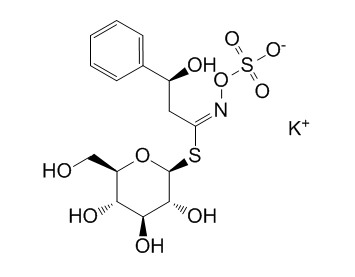
GlucobarbarinCAS# 21087-78-5 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 21087-78-5 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Beige powder |
| Formula | C15H20KNO10S2 | M.Wt | 477.6 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | 2(S)-Hydroxy 2-phenylethylglucosinolate potassium salt | ||
| Solubility | Soluble in water | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Glucobarbarin Dilution Calculator

Glucobarbarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0938 mL | 10.469 mL | 20.938 mL | 41.876 mL | 52.3451 mL |
| 5 mM | 0.4188 mL | 2.0938 mL | 4.1876 mL | 8.3752 mL | 10.469 mL |
| 10 mM | 0.2094 mL | 1.0469 mL | 2.0938 mL | 4.1876 mL | 5.2345 mL |
| 50 mM | 0.0419 mL | 0.2094 mL | 0.4188 mL | 0.8375 mL | 1.0469 mL |
| 100 mM | 0.0209 mL | 0.1047 mL | 0.2094 mL | 0.4188 mL | 0.5235 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Phyllalbine
Catalog No.:BCN8969
CAS No.:4540-25-4
- Epiprogoitrin
Catalog No.:BCN8968
CAS No.:21087-74-1
- 4-Hydroxyglucobrassicin
Catalog No.:BCN8967
CAS No.:83327-20-2
- 4-Methoxyglucobrassicin
Catalog No.:BCN8966
CAS No.:83327-21-3
- Glucoarabin
Catalog No.:BCN8965
CAS No.:67920-64-3
- Glucocamelinin
Catalog No.:BCN8964
CAS No.:67884-10-0
- Glucoraphasatin potassium salt
Catalog No.:BCN8963
CAS No.:245550-64-5
- Glucohirsutin
Catalog No.:BCN8962
CAS No.:21973-60-4
- Gluconasturtiin
Catalog No.:BCN8961
CAS No.:18425-76-8
- Sinalbin potassium salt
Catalog No.:BCN8960
CAS No.:16411-05-5
- Glucocheirolin
Catalog No.:BCN8959
CAS No.:15592-36-6
- Glucobrassicin
Catalog No.:BCN8958
CAS No.:143231-38-3
- Glucolimnanthin
Catalog No.:BCN8971
CAS No.:111810-95-8
- Glucocapparin
Catalog No.:BCN8972
CAS No.:15592-33-3
- Sinalbin
Catalog No.:BCN8973
CAS No.:20196-67-2
- Glucobrassicanapin
Catalog No.:BCN8974
CAS No.:245550-58-7
- Glucoiberin
Catalog No.:BCN8975
CAS No.:15592-34-4
- Glucoberteroin
Catalog No.:BCN8976
CAS No.:245550-65-6
- Glucoerucin
Catalog No.:BCN8977
CAS No.:15592-37-7
- Glucotropaeolin
Catalog No.:BCN8978
CAS No.:5115-71-9
- Glucomoringin
Catalog No.:BCN8979
CAS No.:316165-49-8
- Lupinine
Catalog No.:BCN8981
CAS No.:486-70-4
- Glucoalyssin
Catalog No.:BCN8982
CAS No.:499-37-6
- Neoglucobrassicin potassium salt
Catalog No.:BCN8983
CAS No.:5187-84-8
Aminorex identified in horse urine following consumption of Barbarea vulgaris; a preliminary report.[Pubmed:31890155]
Ir Vet J. 2019 Dec 23;72:15.
Background: Aminorex, (RS)-5- Phenyl-4,5-dihydro-1,3-oxazol-2-amine, is an amphetamine-like anorectic and in the United States a Drug Enforcement Administration [DEA] Schedule 1 controlled substance. Aminorex in horse urine is usually present as a metabolite of Levamisole, an equine anthelmintic and immune stimulant. Recently, Aminorex identifications have been reported in horse urine with no history or evidence of Levamisole administration. Analysis of the urine samples suggested a botanical source, directing attention to the Brassicaceae plant family, with their contained Glucobarbarin and Barbarin as possible sources of Aminorex. Since horsepersons face up to a 1 year suspension and a $10,000.00 fine for an Aminorex identification, the existence of natural sources of Aminorex precursors in equine feedstuffs is of importance to both individual horsepersons and the industry worldwide. Results: Testing the hypothesis that Brassicaceae plants could give rise to Aminorex identifications in equine urine we botanically identified and harvested flowering Kentucky Barbarea vulgaris, ("Yellow Rocket") in May 2018 in Kentucky and administered the plant orally to two horses. Analysis of post-administration urine samples yielded Aminorex, showing that consumption of Kentucky Barbarea vulgaris can give rise to Aminorex identifications in equine urine. Conclusions: Aminorex has been identified in post administration urine samples from horses fed freshly harvested flowering Kentucky Barbarea vulgaris, colloquially "Yellow Rocket". These identifications are consistent with occasional low concentration identifications of Aminorex in equine samples submitted for drug testing. The source of these Aminorex identifications is believed to be the chemically related Barbarin, found as its precursor Glucobarbarin in Kentucky Barbarea vulgaris and related Brassicaceae plants worldwide.
The Role of the Glucosinolate-Myrosinase System in Mediating Greater Resistance of Barbarea verna than B. vulgaris to Mamestra brassicae Larvae.[Pubmed:30218254]
J Chem Ecol. 2018 Dec;44(12):1190-1205.
We investigated the influences of two structurally similar glucosinolates, phenethylglucosinolate (gluconasturtiin, NAS) and its (S)-2-hydroxyl derivative Glucobarbarin (BAR), as well as their hydrolysis products on larvae of the generalist Mamestra brassicae (Lepidoptera: Noctuidae). Previous results suggested a higher defensive activity of BAR than NAS based on resistance toward M. brassicae larvae of natural plant genotypes of Barbarea vulgaris R. Br. (Brassicaceae) dominated by BAR. In the present study, the hypothesis of a higher defensive activity of BAR than NAS was tested by comparing two Barbarea species similarly dominated either by BAR or by NAS and by testing effects of isolated BAR and NAS on larval survival and feeding preferences. Larvae reared on leaf disks of B. verna (Mill.) Asch. had a lower survival than those reared on B. vulgaris P- and G-chemotypes. Leaves of B. verna were dominated by NAS, whereas B. vulgaris chemotypes were dominated by BAR or its epimer. In addition, B. verna leaves showed a threefold higher activity of the glucosinolate-activating myrosinase enzymes. The main product of NAS from breakdown by endogenous enzymes including myrosinases ("autolysis") in B. verna leaves was phenethyl isothiocyanate, while the main products of BAR in autolyzed B. vulgaris leaves were a cyclized isothiocyanate product, namely an oxazolidine-2-thione, and a downstream metabolite, an oxazolidin-2-one. The glucosinolates BAR and NAS were isolated and offered to larvae on disks of cabbage. Both glucosinolates exerted similar negative effects on larval survival but effects of NAS tended to be more detrimental. Low concentrations of BAR, but not of NAS, stimulated larval feeding, whereas high BAR concentrations acted deterrent. NAS only tended to be deterrent at the highest concentration, but the difference was not significant. Recoveries of NAS and BAR on cabbage leaf disks were similar, and when hydrolyzed by mechanical leaf damage, the same isothiocyanate-type products as in Barbarea plants were formed with further conversion of BAR to cyclic products, (R)-5-phenyloxazolidine-2-thione [(R)-barbarin] and (R)-5-phenyloxazolidin-2-one [(R)-resedine]. We conclude that a previously proposed generally higher defensive activity of BAR than NAS to M. brassicae larvae could not be confirmed. Indeed, the higher resistance of NAS-containing B. verna plants may be due to a combined effect of rather high concentrations of NAS and a relatively high myrosinase activity or other plant traits not investigated yet.
Glucosinolate turnover in Brassicales species to an oxazolidin-2-one, formed via the 2-thione and without formation of thioamide.[Pubmed:29886160]
Phytochemistry. 2018 Sep;153:79-93.
Glucosinolates are found in plants of the order Brassicales and hydrolyzed to different breakdown products, particularly after tissue damage. In Barbarea vulgaris R.Br. (Brassicaceae), the dominant glucosinolate in the investigated "G-type" is Glucobarbarin, (S)-2-hydroxy-2-phenylethylglucosinolate. Formation of the nitrile from Glucobarbarin was observed in vitro, while a previously suggested thioamide (synonym thionamide) was not confirmed. Resedine (5-phenyl-1,3-oxazolidin-2-one) was detected after Glucobarbarin hydrolysis in crushed B. vulgaris leaves and siliques, but not in intact parts. The abundance increased for several hours after completion of hydrolysis. The corresponding 1,3-oxazolidine-2-thione (OAT), with the common name barbarin, was also formed, and appeared to be the precursor of resedine. Addition of each of two non-endogenous OATs, (S)-5-ethyl-5-methylOAT and (R)-5-vinylOAT (R-goitrin), to a leaf homogenate resulted in formation of the corresponding 1,3-oxazolidin-2-ones (OAOs), confirming the metabolic connection of OAT to OAO. Formation of OAOs was inhibited by prior brief heating of the homogenate, suggesting enzyme involvement. We suggest the conversion of OATs to OAOs to be catalyzed by an enzyme ("oxazolidinethionase") responsible for turnover of OAT formed in intact plants. Resedine had been reported as an alkaloid from another species - Reseda luteola L. (Resedaceae) - naturally containing the glucosinolate Glucobarbarin. However, resedine was not detected in intact R. luteola plants, but formed after tissue damage. The formation of resedine in two families suggests a broad distribution of putative OATases in the Brassicales; potentially involved in glucosinolate turnover that needs myrosinase activity as the committed step. In agreement with the proposed function of OATase, several candidate genes for myrosinases in glucosinolate turnover in intact plants were discovered in the B. vulgaris genome. We also suggest that biotechnological conversion of OATs to OAOs might improve the nutritional value of Brassicales protein. HPLC-MS/MS methods for detection of these Glucobarbarin products are described.
Glucosinolate hydrolysis products in the crucifer Barbarea vulgaris include a thiazolidine-2-one from a specific phenolic isomer as well as oxazolidine-2-thiones.[Pubmed:25467719]
Phytochemistry. 2015 Jul;115:143-51.
Two isomeric phenolic glucosinolates, m- and p-hydroxyl derivatives of epiGlucobarbarin [(R)-2-hydroxy-2-phenylethylglucosinolate], co-occur in an eastern chemotype (P-type) of the crucifer Barbarea vulgaris along with epiGlucobarbarin itself. Levels of the phenolic derivatives in B. vulgaris were low in summer but higher during fall and winter, allowing isolation of all three glucosinolates. Hydrolysis in vitro, catalyzed by Sinapis alba myrosinase at near neutral pH, resulted in expectable oxazolidine-2-thione type hydrolysis products of epiGlucobarbarin and its m-hydroxyl derivative. In contrast, a thiazolidine-2-one type product was formed in vitro from p-hydroxy epiGlucobarbarin and characterized by UV, IR, MS/MS and 2D NMR. Maceration of leaf material resulted in disappearance of the glucosinolates and formation of the same oxazolidine-2-thione and thiazolidine-2-one products as found in vitro. The detected amounts were comparable to initial amounts of precursor glucosinolates. The corresponding oxazolidine-2-thione type product was also detected quantitatively from Glucobarbarin in foliage of a western genotype (G-type). We suggest that p-hydroxy epiGlucobarbarin is initially converted into the conventional oxazolidine-2-thione, which would further rearrange to a thiazolidine-2-one due to the activating effect of the p-hydroxyl group. We conclude that a subtle difference between isomeric phenolic glucosinolates results in significantly different natural hydrolysis products.
Multiple hydroxyphenethyl glucosinolate isomers and their tandem mass spectrometric distinction in a geographically structured polymorphism in the crucifer Barbarea vulgaris.[Pubmed:25277803]
Phytochemistry. 2015 Jul;115:130-42.
Two distinct glucosinolate (GSL) chemotypes (P and G-types) of Barbarea vulgaris (Brassicaceae) were known from southern Scandinavia, but whether the types were consistent in a wider geographic area was not known. Populations (26) from Eastern and Central Europe were analyzed for GSLs in order to investigate whether the two types were consistent in this area. Most (21) could be attributed to one of the previously described GSL profile types, the P-type (13 populations) and the G-type (8 populations), based on differences in the stereochemistry of 2-hydroxylation, presence or absence of phenolic Glucobarbarin derivatives, and qualitative differences in indole GSL decoration (tested for a subset of 8+6 populations only). The distinction agreed with previous molecular genetic analysis of the same individuals. Geographically, the P-type typically occurred in Eastern Europe while the G-type mainly occurred in Central Europe. Of the remaining five populations, minor deviations were observed in some individuals from two populations genetically assigned to the G-type, and a hybrid population from Finland contained an additional dihydroxyphenethyl GSL isomer attributed to a combinatorial effect of P-type and G-type genes. Major exceptions to the typical GSL profiles were observed in two populations: (1) A G-type population from Slovenia deviated by a high frequency of a known variant in Glucobarbarin biosynthesis ('NAS form') co-occurring with usual G-type individuals. (2) A population from Caucasus exhibited a highly deviating GSL profile dominated by p-hydroxyphenethyl GSL that was insignificant in other accessions, as well as two GSLs investigated by NMR, m-hydroxyphenethylGSL and a partially identified m,p disubstituted hydroxy-methoxy derivative of phenethylGSL. Tandem HPLC-MS of seven NMR-identified desulfoGSLs was carried out and interpreted for increased certainty in peak identification and as a tool for partial structure elucidation. The distinct, geographically separated chemotypes and rare variants are discussed in relation to future taxonomic revision and the genetics and ecology of GSLs in B. vulgaris.
Specific glucosinolate analysis reveals variable levels of epimeric glucobarbarins, dietary precursors of 5-phenyloxazolidine-2-thiones, in watercress types with contrasting chromosome numbers.[Pubmed:25226408]
J Agric Food Chem. 2014 Oct 1;62(39):9586-96.
Watercress obtained in food stores in the United States contained significant levels of epiGlucobarbarin [(R)-2-hydroxy-2-phenylethylglucosinolate] and low levels of the 2S-epimer Glucobarbarin identified by an HPLC+NMR+MS/MS approach. Typical combined levels were 4-7 mumol/g dry wt. The hydrolysis product, 5-phenyloxazolidine-2-thione (barbarin), was detected at similar levels as the precursor glucosinolates after autolysis of fresh watercress in water. Fragmentation patterns in MS(2) of reference desulfoglucosinolates were side chain specific and suitable for routine identification. Watercress was of two main glucosinolate chemotypes: Material from U.S. food stores had a complex profile including Glucobarbarins, gluconasturtiin, indole glucosinolates and high levels (6-28 mumol/g dry wt.) of long-chain methylsulfinylalkyl and methylthioalkyl glucosinolates. Material from European food stores had a simple profile dominated by gluconasturtiin, with low levels of epiGlucobarbarin and moderate levels of indole glucosinolates. Some wild U.S. material was similar to the U.S. food store type. Both types were found to be Nasturtium officinale by floral parts morphology. Cytological analysis of one U.S. food store accession indicated that it represented a chromosome-doubled variant within N. officinale. The nutritional consequences and invasive potential of the U.S. food store chemotype are discussed.
Glucosinolate profile and distribution among plant tissues and phenological stages of field-grown horseradish.[Pubmed:25060759]
Phytochemistry. 2014 Oct;106:178-187.
Profile and distribution of glucosinolates (GLS) were detected in plant tissues of horseradish at different developmental stages: beginning of vegetative re-growth, flowering and silique formation. The GLS profile varied widely in the different tissues: we identified 17 GLS in roots and sprouts, one of which was not previously characterized in horseradish, i.e. the 2(S)-hydroxy-2-phenylethyl-GLS (Glucobarbarin) and/or 2(R)-hydroxy-2-phenylethyl-GLS (epiGlucobarbarin), 11 already found in the roots, including the putative 2-methylsulfonyl-oxo-ethyl-GLS, and 5 previously recognized only in the sprouts. Fifteen of those GLS were also identified in young and cauline leaves, 12 in the mature leaves and 13 in the inflorescences. No difference in GLS profile was observed in plant among the phenological stages. Differences in concentrations of GLS, quantified as desulfated, were found in plant. At the beginning of vegetative re-growth, sprouts while showing the same profile of the roots were much richer in GLS having the highest total GLS concentrations (117.5 and 7.7mumolg(-1) dry weight in sprouts and roots, respectively). During flowering and silique forming stages, the roots still maintained lower amount of total GLS (7.4mumolg(-1) of dry weight, on average) with respect to the epigeous tissues, in which mature and young leaves showed the highest total concentrations (70.5 and 73.8mumolg(-1) of dry weight on average, respectively). Regardless of the phenological stages, the aliphatic GLS were always predominant in all tissues (95%) followed by indolic (2.6%) and benzenic (2.4%) GLS. Sinigrin contributed more than 90% of the total GLS concentration. Aliphatic GLS concentrations were much higher in the epigeous tissues, particularly in the mature and young leaves, while benzenic and indolic GLS concentrations were higher in the roots. Through the phenological stages, GLS concentration increased in young and mature leaves and decreased in cauline leaves and inflorescences, while it remained constant over time in roots.
Acylated glucosinolates with diverse acyl groups investigated by high resolution mass spectrometry and infrared multiphoton dissociation.[Pubmed:24512839]
Phytochemistry. 2014 Apr;100:92-102.
With the aim of developing a procedure for detecting and identifying intact acylated glucosinolates (a-GLSs) found in trace quantities in natural plant samples, extracts of Barbarea vulgaris seeds were analyzed by reversed-phase liquid chromatography coupled with electrospray ionization and Fourier-transform ion cyclotron resonance mass spectrometry (RPLC-ESI FTICR MS). After a preliminary optimization of fragmentation conditions, based on a non-acylated parent glucosinolate (Glucobarbarin) and three previously identified a-GLSs (the 6'-isoferuloyl esters of Glucobarbarin, gluconasturtiin and glucobrassicin), infrared multiphoton dissociation (IRMPD) was employed for a tandem MS-based elucidation of the molecular structures of novel a-GLSs. As a result, three acylated derivatives of Glucobarbarin, esterified at the thioglucose moiety with a coumaric acid isomer, sinapic acid or an isomer and a dimethoxycinnamic acid isomer, were identified. In addition, a further acylated glucosinolate was tentatively identified as the isoferuloyl ester of an unidentified hydroxylic derivative of Glucobarbarin. This is the first demonstration of diversity in the acyl moieties of thioglucose-acylated glucosinolates, which may reflect the substrate specificity of the endogenous acyl transferase. As expected, 6'-isoferuloyl-Glucobarbarin was detected as the main acylated GLS in extracts of B. vulgaris seeds. A quantitative estimate suggested that non-isoferuloyl substituted Glucobarbarins correspond to ca. 0.026% of the level of 6'-isoferuloyl Glucobarbarin. The formation of an uncommon distonic radical anion, most likely generated in the gas phase upon methyl radical (CH3.) loss from the isoferuloyl anion, is demonstrated.
Barbarea vulgaris glucosinolate phenotypes differentially affect performance and preference of two different species of lepidopteran herbivores.[Pubmed:18213497]
J Chem Ecol. 2008 Feb;34(2):121-31.
The composition of secondary metabolites and the nutritional value of a plant both determine herbivore preference and performance. The genetically determined glucosinolate pattern of Barbarea vulgaris can be dominated by either Glucobarbarin (BAR-type) or by gluconasturtiin (NAS-type). Because of the structural differences, these glucosinolates may have different effects on herbivores. We compared the two Barbarea chemotypes with regards to the preference and performance of two lepidopteran herbivores, using Mamestra brassicae as a generalist and Pieris rapae as a specialist. The generalist and specialist herbivores did not prefer either chemotype for oviposition. However, larvae of the generalist M. brassicae preferred to feed and performed best on NAS-type plants. On NAS-type plants, 100% of the M. brassicae larvae survived while growing exponentially, whereas on BAR-type plants, M. brassicae larvae showed little growth and a mortality of 37.5%. In contrast to M. brassicae, the larval preference and performance of the specialist P. rapae was unaffected by plant chemotype. Total levels of glucosinolates, water soluble sugars, and amino acids of B. vulgaris could not explain the poor preference and performance of M. brassicae on BAR-type plants. Our results suggest that difference in glucosinolate chemical structure is responsible for the differential effects of the B. vulgaris chemotypes on the generalist herbivore.
Micellar electrokinetic capillary chromatography--synchronous monitoring of substrate and products in the myrosinase catalysed hydrolysis of glucosinolates.[Pubmed:16806249]
J Chromatogr A. 2006 Oct 20;1130(2):246-52.
A micellar electrokinetic capillary chromatography (MECC) method has been developed for monitoring the myrosinase catalysed hydrolysis of 2-hydroxy substituted glucosinolates and the simultaneous formation of the corresponding degradation products (oxazolidine-2-thiones (OZTs) and nitriles). Glucosibarin ((2R)-2-hydroxy-2-phenylethylglucosinolate) was chosen as the model glucosinolate owing to the difficulties in determining hydrolysis rates of this type of substrates in traditional UV-assays. The method was afterwards validated with Glucobarbarin ((2S)-2-hydroxy-2-phenylethylglucosinolate) and progoitrin ((2R)-2-hydroxybut-3-enylglucosinolate). Aromatic glucosinolates without a 2-hydroxy group in their side chains, such as glucotropaeolin (benzylglucosinolate) and gluconasturtiin (phenethylglucosinolate) were also tested. Formation of the glucosinolate hydrolysis products was monitored simultaneously at 206 nm and 230 nm. This allowed estimation of the extinction coefficient of the OZT derived from glucosibarin, which was found to be 18,000 M(-1) cm(-1) and 12,000 M(-1) cm(-1) at 206 nm and 230 nm, respectively. The developed method has limit of detection of 0.04 mM and 0.06 mM and limit of quantification of 0.2 mM and 0.3 mM for the glucosibarin derived OZT and nitrile, respectively. Linearity of the glucosinolate concentration was examined at six concentration levels from 2.5 mM to 100 mM and at 206 nm a straight line (R(2)=0.9996) was obtained. The number of theoretical plates (N) at the optimal system conditions was 245,000 for the intact glucosibarin, 264,000 for the OZT and 252,000 for the nitrile.
A heritable glucosinolate polymorphism within natural populations of Barbarea vulgaris.[Pubmed:16777152]
Phytochemistry. 2006 Jun;67(12):1214-23.
In natural populations of Barbarea vulgaris we found two distinctly different glucosinolate profiles. The most common glucosinolate profile is dominated (94%) by the hydroxylated form, (S)-2-hydroxy-2-phenylethyl-glucosinolate (Glucobarbarin, BAR-type), whereas in the other type 2-phenylethyl-glucosinolate (gluconasturtiin, NAS-type) was most prominent (82%). NAS-type plants have a 108-fold increase of gluconasturtiin concentration in rosette leaves compared to BAR-type plants. The glucosinolate composition of both chemotypes is consistent throughout all plant organs and after induction with jasmonic acid. Although the glucosinolate profile of the roots has a more diverse composition than other plant organs, it still matches the chemotype. In 12 natural populations that we sampled in Germany, Belgium, France and Switzerland solely BAR-type plants were found. However, eight out of the 15 Dutch populations that were sampled contained 2-22% NAS-type plants. Controlled crosses showed that the chemotype was heritable and determined by a single gene with two alleles. The allele coding for the BAR-type was dominant and the allele for the NAS-type was recessive. The different glucosinolate profiles will yield different hydrolysis products upon damage, and therefore we expect them to differentially affect the multitrophic interactions associated with B. vulgaris in their natural environment.
Sequestration of host plant glucosinolates in the defensive hemolymph of the sawfly Athalia rosae.[Pubmed:11789955]
J Chem Ecol. 2001 Dec;27(12):2505-16.
Interactions between insects and glucosinolate-containing plant species have been investigated for a long time. Although the glucosinolate-myrosinase system is believed to act as a defense mechanism against generalist herbivores and fungi, several specialist insects use these secondary metabolites for host plant finding and acceptance and can handle them physiologically. However, sequestration of glucosinolates in specialist herbivores has been less well studied. Larvae of the tumip sawfly Athalia rosae feed on several glucosinolate-containing plant species. When larvae are disturbed by antagonists, they release one or more small droplets of hemolymph from their integument. This "reflex bleeding" is used as a defense mechanism. Specific glucosinolate analysis, by conversion to desulfoglucosinolates and analysis of these by high-performance liquid chromatography coupled to diode array UV spectroscopy and mass spectrometry, revealed that larvae incorporate and concentrate the plant's characteristic glucosinolates from their hosts. Extracts of larvae that were reared on Sinapis alba contained sinalbin, even when the larvae were first starved for 22 hr and, thus, had empty guts. Hemolymph was analyzed from larvae that were reared on either S. alba, Brassica nigra, or Barbarea stricta. Leaves were analyzed from the same plants the larvae had fed on. Sinalbin (from S. alba), sinigrin (B. nigra), or Glucobarbarin and glucobrassicin (B. stricta) were present in leaves in concentrations less than 1 micromol/g fresh weight, while the same glucosinolates could be detected in the larvae's hemolymph in concentrations between 10 and 31 micromol/g fresh weight, except that glucobrassicin was present only as a trace. In larval feces, only trace amounts of glucosinolates (sinalbin and sinigrin) could be detected. The glucosinolates were likewise found in freshly emerged adults, showing that the sequestered phytochemicals were transferred through the pupal stage.
Oviposition stimulants inBarbarea vulgaris forPieris rapae andP. napi oleracea: isolation, identification and differential activity.[Pubmed:24242065]
J Chem Ecol. 1994 Feb;20(2):423-38.
The closely related butterflies,Pieris rapae andP. napi oleracea, readily laid eggs onBarbarea vulgaris in greenhouse cages. When offered a choice between cabbage andB. vulgaris, P. rapae showed no preference, butP. napi oleracea preferredB. vulgaris. Bioassays of extracts ofB. vulgaris foliage revealed the presence of oviposition deterrent(s) in l-butanol extracts as well as stimulants in the postbutanol water extracts. However, the deterrent effect was apparently outweighed by the strong stimulatory effect in the whole plants. The postbutanol water extract was preferred over an equivalent cabbage extract by both species, but more significantly in the case ofP. napi oleracea. The stimulants were isolated by open column chromatography and HPLC, and the activity was associated with three glucosinolates.P. napi oleracea was more sensitive thanP. rapae to the natural concentration of compounds1 and3, whereas both species were strongly stimulated to oviposit by natural concentrations of compound2. Compounds1 and2 were identified as (2R)-Glucobarbarin and (2S)-Glucobarbarin, respectively, and3 was identified as glucobrassicin, on the basis of their UV, mass, and NMR spectra. When the pure compounds were tested at the same concentrations applied to bean plants, the (2R)-Glucobarbarin at 0.2 mg/plant was preferred over a standard cabbage extract by both butterfly species. However, at a dose of 0.02 mg/plant,P. rapae preferred the cabbage extract whereasP. napi oleracea still preferred the (2R)-Glucobarbarin. No such difference in response of the two species to the same two concentrations of (2S)-Glucobarbarin was obtained. The results indicate a distinct difference in sensitivity of these butterflies to the epimers of Glucobarbarin, and the differences in behavioral responses of the two butterfly species depend to a large extent on the concentration of stimulant present.


