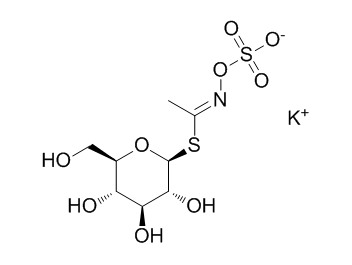
GlucocapparinCAS# 15592-33-3 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 15592-33-3 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | White powder |
| Formula | C8H14KNO9S2 | M.Wt | 371.4 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | Methylglucosinolate potassium salt | ||
| Solubility | Soluble in methanol and water | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Glucocapparin Dilution Calculator

Glucocapparin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6925 mL | 13.4626 mL | 26.9251 mL | 53.8503 mL | 67.3129 mL |
| 5 mM | 0.5385 mL | 2.6925 mL | 5.385 mL | 10.7701 mL | 13.4626 mL |
| 10 mM | 0.2693 mL | 1.3463 mL | 2.6925 mL | 5.385 mL | 6.7313 mL |
| 50 mM | 0.0539 mL | 0.2693 mL | 0.5385 mL | 1.077 mL | 1.3463 mL |
| 100 mM | 0.0269 mL | 0.1346 mL | 0.2693 mL | 0.5385 mL | 0.6731 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Glucolimnanthin
Catalog No.:BCN8971
CAS No.:111810-95-8
- Glucobarbarin
Catalog No.:BCN8970
CAS No.:21087-78-5
- Phyllalbine
Catalog No.:BCN8969
CAS No.:4540-25-4
- Epiprogoitrin
Catalog No.:BCN8968
CAS No.:21087-74-1
- 4-Hydroxyglucobrassicin
Catalog No.:BCN8967
CAS No.:83327-20-2
- 4-Methoxyglucobrassicin
Catalog No.:BCN8966
CAS No.:83327-21-3
- Glucoarabin
Catalog No.:BCN8965
CAS No.:67920-64-3
- Glucocamelinin
Catalog No.:BCN8964
CAS No.:67884-10-0
- Glucoraphasatin potassium salt
Catalog No.:BCN8963
CAS No.:245550-64-5
- Glucohirsutin
Catalog No.:BCN8962
CAS No.:21973-60-4
- Gluconasturtiin
Catalog No.:BCN8961
CAS No.:18425-76-8
- Sinalbin potassium salt
Catalog No.:BCN8960
CAS No.:16411-05-5
- Sinalbin
Catalog No.:BCN8973
CAS No.:20196-67-2
- Glucobrassicanapin
Catalog No.:BCN8974
CAS No.:245550-58-7
- Glucoiberin
Catalog No.:BCN8975
CAS No.:15592-34-4
- Glucoberteroin
Catalog No.:BCN8976
CAS No.:245550-65-6
- Glucoerucin
Catalog No.:BCN8977
CAS No.:15592-37-7
- Glucotropaeolin
Catalog No.:BCN8978
CAS No.:5115-71-9
- Glucomoringin
Catalog No.:BCN8979
CAS No.:316165-49-8
- Lupinine
Catalog No.:BCN8981
CAS No.:486-70-4
- Glucoalyssin
Catalog No.:BCN8982
CAS No.:499-37-6
- Neoglucobrassicin potassium salt
Catalog No.:BCN8983
CAS No.:5187-84-8
- Gluconapin
Catalog No.:BCN8984
CAS No.:245550-57-6
- Progoitrin
Catalog No.:BCN8985
CAS No.:21087-77-4
Effects of Maerua subcordata (Gilg) DeWolf on electrophile-responsive element (EpRE)-mediated gene expression in vitro.[Pubmed:30986264]
PLoS One. 2019 Apr 15;14(4):e0215155.
Plant extracts and phytochemicals may prevent chronic diseases via activation of adaptive cellular stress response pathways including induction of antioxidant and phase II detoxifying enzymes. The regulatory regions of these inducible genes encode the electrophile-response element (EpRE). This study tested the EpRE induction ability of Maerua subcordata (fruit, leaf, root, seed) methanol extracts and selected candidate constituents thereof, identified by liquid chromatography coupled with multistage mass spectroscopy, employing an EpRE luciferase reporter gene assay using hepa-1c1c7 mouse hepatoma cells. A parallel Cytotox CALUX assay using human osteosarcoma U2OS cells was used to monitor any non-specific changes in luciferase activity or cytotoxicity. Results showed that fruit, root, and seed extracts were non-cytotoxic up to a concentration of 30 gram dry weight per litre but the leaf extract exhibited some cytotoxicity and that the leaf (despite some cytotoxicity), fruit, and seed extracts showed strong induction of EpRE mediated gene expression while induction by the root extract was minimal. Selected candidates included glucosinolates, isothiocyanates, and some biogenic amines. Subsequent studies showed that methyl-, ethyl-, isopropyl-, isobutyl- isothiocyanates, and sec-butyl thiocyanate as well as glucobrassicin induced concentration (1-100 muM) dependent EpRE-mediated gene expression while the biogenic amines stachydrine and trigonelline acted as inhibitors of EpRE-mediated gene expression at 100 muM. The identification of glucolepidiin, glucobrassicin, Glucocapparin, stachydrine, and trigonelline in all extracts was confirmed using standards and based on multiple reaction monitoring; yet, glucobrassicin level in the root extract was negligible. In conclusion, this study provided a first report on EpRE mediated gene expression effects of M. subcordata; and despite detection of different glucosinolates in all extracts, those containing glucobrassicin particularly displayed high EpRE induction. Because EpRE inducers are cytoprotective and potential chemopreventive agents while inhibitors are suggested adjuvants of chemotherapy, results of this study imply that process manipulation of this plant may result in herbal preparations that may be used as chemopreventive agents or adjuvants of chemotherapies.
Phytochemical profile and antioxidant activity of caper berries (Capparis spinosa L.): Evaluation of the influence of the fermentation process.[Pubmed:29412927]
Food Chem. 2018 Jun 1;250:54-59.
In this work, we report the phytochemical profile and antioxidant activity of caper berries (Capparis spinosa L.) before and after a fermentation process. The phytochemical profiles were evaluated by high-performance liquid chromatography with UV and electrospray ionization mass spectrometry detection (HPLC-DAD-ESI-MS(n)). Twenty-one compounds were characterized, and seven of them quantified. The main component of non-fermented berries was Glucocapparin, which was degraded upon the fermentation process. Most of the compounds were quercetin and kaempferol glycosides, epicatechin, and proanthocyanidins. The main differences observed upon the fermentation process were a decrease in epicatechin concentration, the hydrolysis of quercetin glycosides, and the degradation of glucosinolates. Total phenolic and flavonoid contents, as well as the antioxidant activities by the in vitro antioxidant assays DPPH and ABTS(+), were determined, observing that the values were slightly higher after the fermentation process.
Metabolomic study of wild and cultivated caper (Capparis spinosa L.) from different areas of Sardinia and their comparative evaluation.[Pubmed:27489055]
J Mass Spectrom. 2016 Sep;51(9):716-28.
Capparis spinosa L. (Capparidaceae), also known as caper, is widely known for its very aromatic flower buds (capers),that are largely employed as a flavouring in cooking. Capparis species are regarded as a potential source of important bioactive compounds, in fact, due to their botanical relationship with Brassica species; they contain glucosinolates, secondary plant metabolites, that have been studied for their potential anticarcinogenic properties. In addition, the presence of other numerous beneficial compounds such as polyphenols, alkaloids, lipids, vitamins and minerals have been reported. The aim of this study was to individuate and determinate the principal bioactive compounds occurring in different part (leaves, buds and flowers) of wild and cultivated C. spinosa collected from different area of Sardinia (Italy). Ultra-high performance liquid chromatography-triple quadrupole/linear ion trap tandem mass spectrometry methods were used for identification and simultaneous determination of 27 bioactive molecules. Analysis of different samples revealed qualitative and quantitative differences in the content of flavonoids, glucosinolates, anthocyanins and phenolic acids. In particular, Glucocapparin resulted the most abundant with values ranging from 112 to 364 mg/100 g Fresh Weight (FW); followed by rutin with highest value of 126 mg/100 g FW, 4-hydroxyglucobrassicin with highest value of 42 mg/100 g FW and isorhamnetin 3-O-rutinoside with highest value of 24 mg/100 g FW. Based on this metabolomic targeted approach, quantitative results were treated by principal component analysis to explore and visualise correlation and discrimination among collections of C. spinosa samples. Copyright (c) 2016 John Wiley & Sons, Ltd.
Inhibitory Effect on Lipid Absorption and Variability of Chemical Constituents from Capparis sicula subsp. sicula and Capparis orientalis.[Pubmed:27138247]
Chem Biodivers. 2016 Jun;13(6):755-61.
In continuation of our research program on Mediterranean dietary plants, a bioassay-guided fractionation of extracts from several accessions of Capparis sicula subsp. sicula and Capparis orientalis aerial parts was carried out. Antilipidemic activity of samples was assayed using inhibition of pancreatic lipase. To study the metabolic variability in Capparis species, HPTLC analyses were performed in order to characterize the species through the detection, isolation, and quantitative evaluation of rutin taken as significant chemical marker. The best activity was exerted by C. orientalis accession no. C10 and C. sicula subsp. sicula accession no. C6. The bioactivity evaluation of specific chemical markers, rutin and Glucocapparin, led to the identification of a potent antilipidemic compound rutin. The HPTLC analysis showed large variation among the different analyzed samples with respect to rutin concentration. The chemical investigation showed a different composition between the species and between the collection zones. The variations showed by the studied accessions of caper could be attributed to exogenous factors. Capparis species contained predominantly quercetin rutinoside (rutin), accompanied by other constituents such as the glucosinolate Glucocapparin. These rutin-rich extracts exhibited pronounced dose-dependent enzyme inhibitory activities toward pancreatic lipase.
Identification of glucosinolates in capers by LC-ESI-hybrid linear ion trap with Fourier transform ion cyclotron resonance mass spectrometry (LC-ESI-LTQ-FTICR MS) and infrared multiphoton dissociation.[Pubmed:22972784]
J Mass Spectrom. 2012 Sep;47(9):1160-9.
An liquid chromatography-mass spectrometry method using electrospray ionization in negative ion mode coupled with a hybrid quadrupole linear ion trap and Fourier transform ion cyclotron resonance (FTICR) mass spectrometer was applied to characterize of intact glucosinolates (GLSs) in crude sample extracts of wild bud flowers of Capparis spinosa (Capparis species, family Capparaceae). Structural information of GLSs was obtained upon precursor ions' isolation within the FTICR trapping cell and subsequent fragmentation induced by infrared multiphoton dissociation (IRMPD). Such a fragmentation was found very useful in terms of chemical identification of all precursor ions [M-H](-) including sulfur-rich GLSs reported here for the first time. Along with most common GLSs already found in capers such as Glucocapparin, isopropyl/n-propyl-GLS, mercapto-Glucocapparin, and two indolic GLS, i.e., 4-hydroxyglucobrassicin and glucobrassicin, the occurrence of the uncommon glycinyl-Glucocapparin as well as two sulfur-rich GLSs is reported. IRMPD showed an increased selectivity towards disulfide bond cleavages with thiol migration, suggesting the side chain structure of non-targeted compounds, i.e., disulfanyl-Glucocapparin and trisulfanyl-Glucocapparin. Glucocapparin [2.05 +/- 0.25 mg/g, dry weight (dw)] was the most abundant GLS, followed by glucobrassicin (232 +/- 18 microg/g, dw) and 4-hydroxyglucobrassicin (89 +/- 12 microg/g, dw). All other compounds were present at very low content ranging from 0.5 to 1.5 microg/g dw.
The anticarcinogenic potential of essential oil and aqueous infusion from caper (Capparis spinosa L.).[Pubmed:26434289]
Food Chem. 2012 May 1;132(1):261-7.
The present study assessed the influence of essential oil and aqueous infusion from wild-grown caper (Capparis spinosa L.) on cell growth, NF-kappaB activation, apoptosis and cell cycle in the human colon carcinoma cell line, HT-29. Methyl isothiocyanate (92.06%), a degradation product of glucosinolate Glucocapparin, was detected as major component of essential oil from caper leaves and flower buds. Aqueous infusion of caper showed an interesting and variegate compositional pattern containing several phenolic compounds, among which a flavonol glycoside, rutin (quercetin 3-O-rutinoside, 50.7%) and 5-caffeoyl-quinic acid (chlorogenic acid, 17.5%) were detected as dominant. Caper essential oil and aqueous infusion showed time- and dose-dependent high inhibitory effect on HT-29 cell proliferation. In addition, they induced the inhibition on nuclear factor kappaB (NF-kappaB) activity in a dose-dependent manner, while they did not show any effect on apoptosis in HT-29 cells. Flow cytometric analysis indicated that treatment with caper essential oil and aqueous infusion resulted in G2/M cell cycle arrest in a dose-dependent manner. Presented results suggest that caper contains volatile and non-volatile compounds which potentially can play an important role in colon cancer prevention.
The influence of collection zone on glucosinolates, polyphenols and flavonoids contents and biological profiles of Capparis sicula ssp. sicula.[Pubmed:21436235]
Food Sci Technol Int. 2011 Apr;17(2):87-97.
This study aimed to evaluate the influence of collection zone on total phenol, flavonoid and glucosinolate contents and antioxidant and anti-inflammatory activities of caper (Capparis sicula ssp. sicula). This species has been characterized through the detection, isolation and quantitative evaluation of chemical markers (polyphenols, flavonoids and glucosinolates). The chemical investigation showed a different composition between the two collection zones. While the total amounts of phenolics and flavonoids of the two samples were quite the same, their high-performance liquid chromatography profiles were very different. In both samples, the most abundant aglycone was quercetin which accounted for 60% of total flavonoids. Nuclear magnetic resonance data analysis allowed the identification of two compounds: 3,5-dicaffeoylquinic and 4,5-dicaffeoylquinic acids which represented 6.67% and 15.94%, respectively, of the total amount of flavonoids in sample 1. In sample 2, these two acids were still present, but their percentages were much less (2.20% and 1.71%, respectively). As far as we know, this is the first report about the presence of dicaffeoylquinic acids in Capparis. With regard to glucosinolate content, sample 1 showed a higher content of glucosinolates. In both samples, Glucocapparin was the most abundant compound. Antioxidant activity of the methanolic C. sicula extracts using diphenyl picrylhydrazyl, beta-carotene bleaching test and oxygen radical absorbance capacity showed that the sample 2 was more active than 1. As regards the inhibition of NO production, the extracts from sample 2 were more active than those from sample 1.
Glucosinolates of seven medicinal plants from Thailand.[Pubmed:12048016]
Fitoterapia. 2002 Jun;73(3):209-16.
Nasturtium montanum was shown to contain glucobrassicin, 9-methylthionyl glucosinolate, oct-7-enyl glucosinolate, non-7-enyl glucosinolate, dec-7-enyl glucosinolate, methylsulfonyloctyl glucosinolate, methylsulfonylnonyl glucosinolate, methylsulfonyldecyl glucosinolate, benzyl glucosinolate, and Cleome chelidonii contained Glucocapparin and glucocleomin. Raphanus sativus contained sulforaphene, plus sulforaphane, glucodehydroerucin, and gluconapin; Lepidum sativum contained benzyl glucosinolate and glucotropaeolin; Eruca versicaria contained glucoerucin; Cleome viscosa contained Glucocapparin and glucocleomin, while Gynandropsis gynandra contained Glucocapparin.
Biological activity of the shrubBoscia senegalensis (PERS.) LAM. ex Poir. (Capparaceae) on stored grain insects.[Pubmed:24248882]
J Chem Ecol. 1993 Feb;19(2):377-89.
Biological activity of leaves, fruits and extract of the African shrubBoscia senegalensis (PERS.) LAM. ex Poir. was evaluated against five stored-grain insects. When added to cowpeas at 2-4% (w/w), fresh ground fruits and leaves caused 80-100% mortality inCallosobruchus maculants (F.) adults and significantly reduced both emergence and damage of the F1 progeny. Acetone fruit extract exhibited a potent fumigant effect onProstephanus truncatus HORN, C.maculatus, andSitotroga cerealella OLIV.; with LT50 values of 3.8, 2.3, and below 1.5 hr, respectively. LC50 determination forB. senegalensis fruits and leaves as well as pure methylisothiocyanate (MITC) onTribolium castaneum HERBST,Sitophilus zeamais MOTSCH. andC. maculatus showed a differential response of the insects to plant parts or MITC. Quantitative dosage ofBoscia active components and LC50 values obtained for the plant tissues, compared to those of pure molecules, indicate that the biological activity ofB. senegalensis is due to the liberation of MITC from a glucosinolate precursor Glucocapparin contained inBoscia fruits and leaves.
Glucocapparin variability among four populations ofIsomeris arborea Nutt.[Pubmed:24276006]
J Chem Ecol. 1988 Feb;14(2):623-33.
Glucocapparin (methylglucosinolate), a putative defense compound, was found to vary between desert and nondesert populations ofIsomeris arborea (Capparaceae): Plants from desert populations contained greater concentrations than nondesert plants in four of the five organs analyzed. Immature leaves at desert sites had average Glucocapparin concentrations of 9.2 mg/g and 8.4 mg/g, while nondesert sites averaged 6.0 mg/g and 4.6 mg/g. Mature leaves from desert sites had average concentrations of 12.8 mg/g and 7.9 mg/g; leaves from plants at nondesert sites contained approximately one third to one half of those concentrations. A similar pattern was observed in capsule walls and seeds but not in flower buds; for these, non-desert plants contained a slightly higher concentration of Glucocapparin. Our studies show that nitrogen and Glucocapparin concentrations fluctuate throughout the year and contribute to the observed variability among populations during any particular season. Glucocapparin may fluctuate seasonally as much as 37% in immature leaves and 78% in mature leaves. In a controlled experiment, Glucocapparin concentration varied inversely with nitrogen fertilizer treatment. The plants treated with fertilizer lacking nitrogen ranged from 10.1 mg/g to 10.9 mg/g glucapparin, which was roughly twice the concentration of those supplied with 20 mM nitrogen in the fertilizer.
Isolation and characterization of glucocapparin inIsomeris arborea nutt.[Pubmed:24307123]
J Chem Ecol. 1986 Jun;12(6):1449-58.
Isomeris arborea (Capparaceae), is the only woody caper endemic to southern California and northern Baja. Methylglucosinolate, also known as Glucocapparin, was the only glucosinolate found inI. arborea organs by paper chromatography of the thiourea derivatives and was quantitatively determined by gas chromatography by hydrolytic products. The concentration of Glucocapparin ranged from an average of 4.6 mg/g wet weight in mature leaves to 5.2 mg/g wet weight in immature leaves. Buds averaged 6.2 mg/g wet weight and capsule walls 1.8 mg/g wet weight. Seeds contained an average of 14.3 mg/g wet weight of Glucocapparin. Glucocapparin concentration was found to vary significantly among the mature leaves of individuals within a single population. This compound is known to be deleterious to nonadapted herbivores and may be implicated in the chemical defense mechanism ofI. arborea.


