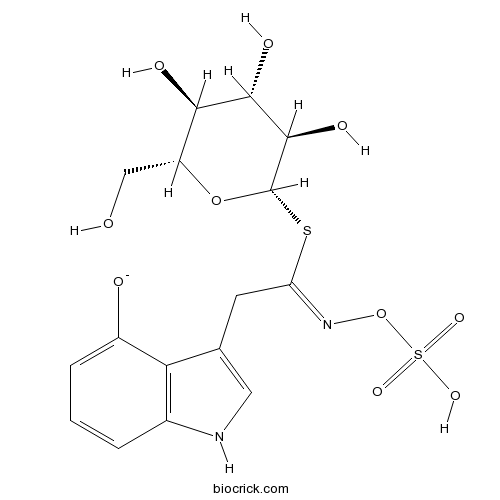
4-HydroxyglucobrassicinCAS# 83327-20-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 83327-20-2 | SDF | Download SDF |
| PubChem ID | 138402395 | Appearance | Powder |
| Formula | C16H19KN2O10S2 | M.Wt | 502.6 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-[(2Z)-2-sulfooxyimino-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]sulfanylethyl]-1H-indol-4-olate | ||
| SMILES | C1=CC2=C(C(=C1)[O-])C(=CN2)CC(=NOS(=O)(=O)O)SC3C(C(C(C(O3)CO)O)O)O | ||
| Standard InChIKey | CSMYCLLHRFFFLG-WVGMDVCISA-M | ||
| Standard InChI | InChI=1S/C16H20N2O10S2/c19-6-10-13(21)14(22)15(23)16(27-10)29-11(18-28-30(24,25)26)4-7-5-17-8-2-1-3-9(20)12(7)8/h1-3,5,10,13-17,19-23H,4,6H2,(H,24,25,26)/p-1/b18-11-/t10-,13-,14+,15-,16+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

4-Hydroxyglucobrassicin Dilution Calculator

4-Hydroxyglucobrassicin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9897 mL | 9.9483 mL | 19.8965 mL | 39.7931 mL | 49.7413 mL |
| 5 mM | 0.3979 mL | 1.9897 mL | 3.9793 mL | 7.9586 mL | 9.9483 mL |
| 10 mM | 0.199 mL | 0.9948 mL | 1.9897 mL | 3.9793 mL | 4.9741 mL |
| 50 mM | 0.0398 mL | 0.199 mL | 0.3979 mL | 0.7959 mL | 0.9948 mL |
| 100 mM | 0.0199 mL | 0.0995 mL | 0.199 mL | 0.3979 mL | 0.4974 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-Methoxyglucobrassicin
Catalog No.:BCN8966
CAS No.:83327-21-3
- Glucoarabin
Catalog No.:BCN8965
CAS No.:67920-64-3
- Glucocamelinin
Catalog No.:BCN8964
CAS No.:67884-10-0
- Glucoraphasatin potassium salt
Catalog No.:BCN8963
CAS No.:245550-64-5
- Glucohirsutin
Catalog No.:BCN8962
CAS No.:21973-60-4
- Gluconasturtiin
Catalog No.:BCN8961
CAS No.:18425-76-8
- Sinalbin potassium salt
Catalog No.:BCN8960
CAS No.:16411-05-5
- Glucocheirolin
Catalog No.:BCN8959
CAS No.:15592-36-6
- Glucobrassicin
Catalog No.:BCN8958
CAS No.:143231-38-3
- Glucoraphasatin
Catalog No.:BCN8957
CAS No.:28463-23-2
- Noratropine
Catalog No.:BCN8955
CAS No.:16839-98-8
- Scopolamine N-oxide hydrobromide
Catalog No.:BCN8953
CAS No.:6106-81-6
- Epiprogoitrin
Catalog No.:BCN8968
CAS No.:21087-74-1
- Phyllalbine
Catalog No.:BCN8969
CAS No.:4540-25-4
- Glucobarbarin
Catalog No.:BCN8970
CAS No.:21087-78-5
- Glucolimnanthin
Catalog No.:BCN8971
CAS No.:111810-95-8
- Glucocapparin
Catalog No.:BCN8972
CAS No.:15592-33-3
- Sinalbin
Catalog No.:BCN8973
CAS No.:20196-67-2
- Glucobrassicanapin
Catalog No.:BCN8974
CAS No.:245550-58-7
- Glucoiberin
Catalog No.:BCN8975
CAS No.:15592-34-4
- Glucoberteroin
Catalog No.:BCN8976
CAS No.:245550-65-6
- Glucoerucin
Catalog No.:BCN8977
CAS No.:15592-37-7
- Glucotropaeolin
Catalog No.:BCN8978
CAS No.:5115-71-9
- Glucomoringin
Catalog No.:BCN8979
CAS No.:316165-49-8
Profiling of Individual Desulfo-Glucosinolate Content in Cabbage Head (Brassica oleracea var. capitata) Germplasm.[Pubmed:32316621]
Molecules. 2020 Apr 17;25(8). pii: molecules25081860.
Individual glucosinolates (GSLs) were assessed to select cabbage genotypes for a potential breeding program. One hundred forty-six cabbage genotypes from different origins were grown in an open field from March to June 2019; the cabbage heads were used for GSL analyses. Seven aliphatics [glucoiberin (GIB), progoitrin (PRO), epi-progoitrin (EPI), sinigrin (SIN), glucoraphanin (GRA), glucoerucin (GER) and gluconapin (GNA)], one aromatic [gluconasturtiin (GNS)] and four indolyl GSLs [glucobrassicin (GBS), 4-Hydroxyglucobrassicin (4HGBS), 4-methoxyglucobrassicin (4MGBS), neoglucobrassicin (NGBS)] were found this study. Significant variation was observed in the individual GSL content and in each class of GSLs among the cabbage genotypes. Aliphatic GSLs were predominant (58.5%) among the total GSLs, followed by indolyl GSL (40.7%) and aromatic GSLs (0.8%), showing 46.4, 51.2 and 137.8% coefficients of variation, respectively. GIB, GBS and NGBS were the most common GSLs found in all genotypes. GBS was the most dominant GSL, with an average value of 3.91 micromol g(-1) (0.79 to 13.14 micromol g(-1)). SIN, GIB, PRO and GRA were the other major GSLs, showing average values of 3.45, 1.50, 0.77 and 0.62 micromol g(-1), respectively. The genotypes with relatively high contents of GBS, SIN, GIB and GRA warrant detailed studies for future breeding programs since the hydrolysis products of these GSLs have several anti-cancer properties.
Investigation of the glucosinolates in Hesperis matronalis L. and Hesperis laciniata All.: Unveiling 4'-O-beta-d-apiofuranosylglucomatronalin.[Pubmed:31918339]
Carbohydr Res. 2020 Feb;488:107898.
The glucosinolate (GSL) profiles of wild-growing plants from the genus Hesperis, i.e. Hesperis laciniata All. (leaf, stem, flower, and root) from Croatia and Hesperis matronalis L. (leaf, stem, flower, seed, and root) from Canada, were established by LC-MS. During this investigation, 5-(methylsulfanyl)pentyl- (3), 6-(methylsulfanyl)hexyl- (4), 6-(methylsulfinyl)hexyl- (6), and 4'-alpha-l-rhamnopyranosyloxybenzyl- (17) GSLs were identified. In addition, the presence of 7-(methylsulfinyl)heptyl GSL (18), hydroxy-(alpha-l-rhamnopyranosyloxy)benzyl GSL, and of one d-apiosylated analogue of 17 were suggested. Moreover, one new GSL, 4'-O-beta-d-apiofuranosylglucomatronalin (19) was isolated from H. laciniata (flower, steam and leaf) and characterized by spectroscopic data interpretation. Finally, we report the presence of 3, 4, 6, 19, glucosinalbin (12), and 4-Hydroxyglucobrassicin (20) in H. matronalis and hypothesize the presence of glucomatronalin (13) and 3-hydroxy-6-(methylsulfanyl)hexyl GSL (21).
Comparative analysis of glucosinolate production in hairy roots of green and red kale (Brassica oleracea var. acephala).[Pubmed:31124740]
Prep Biochem Biotechnol. 2019;49(8):775-782.
Glucosinolates (GSLs) are sulfur- and nitrogen-containing secondary metabolites that function in plant defense and provide benefits to human health. In this study, using Agrobacterium rhizogenes R1000, green and red kale hairy roots were established. The expression levels of GSLs biosynthesis genes and their accumulation in both kale hairy roots were analyzed by quantitative real-time PCR and HPLC. The results showed that the expression of most indolic GSLs biosynthesis genes was higher in the hairy roots of green kale than in that of red kale. In contrast, the expression of BoCYP83A1 and BoSUR1 encoding key enzymes aromatic GSL biosynthesis was significantly higher in red kale hairy root. The HPLC analysis identified six GSLs. The levels of 4-methoxyglucobrassicin, glucobrassicin, and 4-Hydroxyglucobrassicin were 6.21, 5.98, and 2 times higher, respectively, in green kale than in red kale, whereas the levels of neoglucobrassicin and gluconasturtiin were 16.2 and 3.48 times higher, respectively, in red kale than in green kale. Our study provides insights into the underlying mechanisms of GSLs biosynthesis in kale hairy roots and can be potentially used as "biological factories" for producing bioactive substances such as GSLs.
Variation of glucosinolates on position orders of flower buds in turnip rape (Brassica rapa).[Pubmed:30174493]
Saudi J Biol Sci. 2017 Nov;24(7):1562-1566.
To glucosinolate (GSL) contents on flower buds depending on their position orders in turnip rape (Brassica rapa), three Japanese 'Nabana' cultivars such as cv. No. 21 (Brassica rapa, early type), cv. Husanohana (B. rapa, late type) and cv. Norin No. 20 (B. napus) were investigated using HPLC analysis. Ten GSLs including glucoraphanin, sinigrin, glucoalyssin, napoleiferin, gluconapin, 4-Hydroxyglucobrassicin, glucobrassicanapin, glucobrassicin, and gluconasturtiin were detected. Differences in individual and total GSL contents were found between two plant varieties, which are also depending on various developmental stages. Among the GSLs, gluconapin (mean 23.11 mumole/g dry weight (DW) and glucobrassicanapin (mean 13.41 mumole/g DW) documented the most abundant compounds and contributed average 39 and 27% of the total GSLs, but indolyl and aromatic GSLs together accounted >10% of the total GSLs. The presence of significant quantities of gluconapin in the cultivars should be studied more extensively, since the GSL is mainly responsible for the bitter taste.
Influence of silver nanoparticles on the enhancement and transcriptional changes of glucosinolates and phenolic compounds in genetically transformed root cultures of Brassica rapa ssp. rapa.[Pubmed:30056602]
Bioprocess Biosyst Eng. 2018 Nov;41(11):1665-1677.
Glucosinolates (GSLs) and phenolic compounds (PCs) are biologically active and involved in the defense reaction of plants; these compounds have a beneficial effect on human health. In this study, we described the influence of biologically synthesized silver nanoparticles (Ag NPs) to enhance the phytochemicals (GSLs and PCs), their transcription levels, and their biological activities in genetically transformed root cultures (hairy root cultures) of Brassica rapa. The concentrations of silver and reactive oxygen species (malondialdehyde and hydrogen peroxide) were highly elevated in the Ag NP-elicited hairy roots (HRs). Glucosinolates (glucoallysin, glucobrassicanapin, sinigrin, progoitrin, gluconapin, 4-methoxyglucobrassicin, 4-Hydroxyglucobrassicin, glucobrassicin, neoglucobrassicin, and gluconasturtiin) and their transcripts (MYB34, MYB51, MYB28, and MYB29) were significantly enhanced in the Ag NP-elicited HRs. Moreover, the phenolic compounds (flavonols, hydroxybenzoic, and hydroxycinnamic acids) were significantly enriched in the Ag NP-elicited HRs. Total phenolic and flavonoid concentrations and their transcripts (PAL, CHI, and FLS) were higher in the Ag NP-elicited HRs than in the non-elicited HRs. Additionally, biological (antioxidant, antimicrobial, and anticancer) activities were significantly higher in the Ag NP-elicited HRs than in the non-elicited HRs. The Ag NP-elicited HR cultures offered an efficient and promising in vitro method to increase the production of health-promoting bioactive compounds, which may be useful in nutraceutical and pharmaceutical industries.
Formation of Sulforaphane and Iberin Products from Thai Cabbage Fermented by Myrosinase-Positive Bacteria.[Pubmed:29671807]
Molecules. 2018 Apr 19;23(4). pii: molecules23040955.
Myrosinase-positive bacteria from local fermented foods and beverages in Thailand with the capacity to metabolize glucosinolate and produce isothiocyanates (ITCs) were isolated and used as selected strains for Thai cabbage fermentation. Enterobacter xiangfangensis 4A-2A3.1 (EX) from fermented fish and Enterococcus casseliflavus SB2X2 (EC) from fermented cabbage were the two highest ITC producers among seventeen strains identified by 16S rRNA technique. EC and EX were used to ferment Thai cabbage (Brassica oleracea L. var. capitata) containing glucoiberin, glucoraphanin and 4-Hydroxyglucobrassicin at 430.5, 615.1 and 108.5 micromol/100 g DW, respectively for 3 days at 25 degrees C. Different amounts of iberin nitrile, iberin, sulforaphane and indole 3-acetonitrile were produced by spontaneous, EX- and EC-induced cabbage fermentations, and significantly higher ITCs were detected (p < 0.01) with increased antioxidant activities. Iberin and sulforaphane production in EX-induced treatment peaked on day 2 at 117.4 and 294.1 micromol/100 g DW, respectively, significantly higher than iberin at 51.7 micromol/100 g DW but not significantly higher than sulforaphane at 242.6 micromol/100 g DW in EC-induced treatment at day 2. Maximum health-promoting benefits from this functional food can be obtained from consumption of a liquid portion of the fermented cabbage with higher ITC level along with a solid portion.
Molecular characterization of glucosinolates and carotenoid biosynthetic genes in Chinese cabbage (Brassica rapa L. ssp. pekinensis).[Pubmed:29379360]
Saudi J Biol Sci. 2018 Jan;25(1):71-82.
The present study aimed to investigate the contents of glucosinolates (GSLs) and carotenoids in eleven varieties of Chinese cabbage in relation to the expression level of the important transcription factors. MS and HPLC analysis identified the presence of 13 GSLs (progoitrin, sinigrin, glucoalyssin, gluconapoleiferin, gluconapin, glucocochlearin, glucobrassicanapin, glucoerucin, 4-Hydroxyglucobrassicin, glucobrassicin, 4-methoxyglucobrassicin, neoglucobrassicin and gluconasturtiin) and four carotenoids (lutein, zeaxanthin, alpha-carotene and beta-carotene). GSL contents were varied among the different cabbage varieties. The total GSL content ranged from 2.7 to 57.88 mumol/g DW. The proportion of gluconapin (54%) and glucobrassicanapin (22%) was higher in all the varieties, respectively. Results documented the variation in total and individual carotenoid contents that have also been observed among different varieties; however, the total carotenoid contents ranged from 289.12 to 1001.41 mg kg(-1) DW (mean 467.66). Interestingly, the proportion of lutein (66.5) and beta-carotene (25.9) were higher than alpha-carotene (5.1) and zeaxanthin (2.5%). Consequently, the expression level of the regulatory gene, MYB28 was higher in 'K0648' and was directly proportional to GSL content. Similarly, the expression levels of 1-PSY were higher in 'K0112'; however, the expression levels of 2-ZDS, 3-LCYB, 4-LCYE, 5-CHXB and 7-NCED genes showed no significant difference. In addition, the correlation between GSL and carotenoid contents and gene expression level showed moderate significant difference in each Chinese cabbage.
Glucosinolate Profiles in Cabbage Genotypes Influence the Preferential Feeding of Diamondback Moth (Plutella xylostella).[Pubmed:28769953]
Front Plant Sci. 2017 Jul 18;8:1244.
Diamondback moth (DBM), Plutella xylostella L., is a devastating pest of cabbage worldwide whose feeding attributes are influenced by glucosinolate profiles of the plant. Identifying the specific glucosinolates associated with plants' resistance mechanism can provide cues to novel points of intervention in developing resistant cultivars. We studied the DBM larval feeding preference and extent of damage on cabbage leaves via controlled glass-house and in vitro multiple- and two-choice feeding tests. These feeding attributes were associated with the individual glucosinolate profiles, analyzed by HPLC, of each of the eight cabbage genotypes using multivariate analytical approach to identify the glucosinolates that may have roles in resistance. Both the glass-house and in vitro multiple-choice feeding tests identified the genotype BN4303, BN4059, and BN4072 as the least preferred (resistant) and Rubra, YR Gold and BN3383 as most preferred (susceptible) genotypes by DBM larvae. The principal component analysis separated the genotypes based on lower feeding scores in association with higher contents of glucobrassicin, glucoiberin, glucoiberverin in one direction and 4-Hydroxyglucobrassicin, glucoerucin, glucoraphanin, and progoitrin in opposite direction in a way to explain the major variation in resistant versus susceptible genotypes based on their extent of preference and leaf area damage. The simultaneous presence (or higher contents) of glucobrassicin, glucoiberin, and glucoiberverin and the absence (or lower contents) of 4-Hydroxyglucobrassicin, glucoerucin, glucoraphanin, and progoitrin in the least preferred genotypes and vice-versa in most preferred genotypes indicated their apparent role as putative repellents and attractants of DBM larvae in cabbage genotypes, respectively. These novel findings add to the current knowledgebase on the roles of glucosinolates in plant-herbivore interactions and will be helpful in setting breeding priorities for improving the resistance against DBM in cabbage using conventional and biotechnological approaches.
Identification of Glucosinolates in Seeds of Three Brassicaceae Species Known to Hyperaccumulate Heavy Metals.[Pubmed:27981800]
Chem Biodivers. 2017 Mar;14(3).
Plants from the Brassicaceae family are known to contain secondary metabolites called glucosinolates. Our goal was to establish by LC/MS the glucosinolate profile of seeds of three Brassicaceae species known to hyperaccumulate heavy metals. We investigated Alyssum fallacinum auct. non Hausskn., Iberis intermedia Guers., and Noccaea caerulescens (J. Presl & C. Presl) F. K. Mey. Our results indicate that A. fallacinum seeds contain glucoiberin and glucoibervirin, which had not been previously identified in this plant. Furthermore, we report for the first time the presence of glucoiberin, glucoibervirin, glucotropaeolin, and sinigrin in I. intermedia. We have detected for the first time glucoconringiin in N. caerulescens. In addition, glucosinalbin, 4-Hydroxyglucobrassicin, and glucomoringin were also detected.
Effects of seed priming, salinity and methyl jasmonate treatment on bioactive composition of Brassica oleracea var. capitata (white and red varieties) sprouts.[Pubmed:27625158]
J Sci Food Agric. 2017 Jun;97(8):2291-2299.
BACKGROUND: Brassica spp. sprouts are rich in nutrients and bioactive compounds, especially glucosinolates and phenolic acid derivatives, and the composition of these young germinating seeds can be altered by several external factors. In this study two cabbage varieties (Brassica oleracea var. capitata, red and white) were studied using seed priming (KCl 50 mmol L(-1) ; NaCl 150 mmol L(-1) ) and MeJA spraying (25 micromol L(-1) ) to elicit the phytochemical content of edible sprouts. RESULTS: The red variety was richer in glucosinolates and phenolic compounds than the white one but not in mineral nutrients. Seed priming enhanced the potassium (K) content and flavonols in both varieties, while the total content of glucosinolates was reduced after seed priming only in the red variety. The white variety responded better than the red one to KCl seed priming, increasing the flavonols (89%). Salinity did not induce any change in the phytochemical content of these two varieties. Elicitation with sprayed MeJA was effective in significantly increasing the content of indolic glucosinolates glucobrassicin (5.7-fold) and neoglucobrassicin (9.7-fold) in the red cultivar. In the white variety, in addition to glucobrassicin (19.4-fold) and neoglucobrassicin (9.4-fold), 4-Hydroxyglucobrassicin (2.3-fold) was also enhanced. MeJA also elicited significant amounts of anthocyanins (41%) and chlorogenic acid derivatives (329%) in the white variety. CONCLUSION: KCl seed priming and MeJA elicitation promoted the phytochemical composition of the cabbage varieties, especially in the white variety. The application of NaCl resulted in less efficient elicitation. (c) 2016 Society of Chemical Industry.
Mechanism Underlying the Onset of Internal Blue Discoloration in Japanese Radish (Raphanus sativus) Roots.[Pubmed:27530819]
J Agric Food Chem. 2016 Sep 7;64(35):6745-51.
The internal blue discoloration observed in Japanese radish (Raphanus sativus L.) roots is a physiological phenomenon caused by storage following harvest at approximately 20 degrees C and poses a serious problem for farmers. Here, we describe the mechanism underlying the onset of internal blue discoloration of three cultivars: Hukuhomare, SC8-260, and Yuto. Each cultivar was maintained under the same conditions. Additionally, Hukuhomare radish roots were maintained at three different cultivation conditions in a related experiment. The blue discoloration in radish roots was caused by the oxidation of 4-Hydroxyglucobrassicin as a result of an increase in oxidative stress involving peroxidase. Thus, the extent of blue discoloration was influenced by the chemical balance involving 4-Hydroxyglucobrassicin content, antioxidant capacity, and oxidation activity.
Metabolomic study of wild and cultivated caper (Capparis spinosa L.) from different areas of Sardinia and their comparative evaluation.[Pubmed:27489055]
J Mass Spectrom. 2016 Sep;51(9):716-28.
Capparis spinosa L. (Capparidaceae), also known as caper, is widely known for its very aromatic flower buds (capers),that are largely employed as a flavouring in cooking. Capparis species are regarded as a potential source of important bioactive compounds, in fact, due to their botanical relationship with Brassica species; they contain glucosinolates, secondary plant metabolites, that have been studied for their potential anticarcinogenic properties. In addition, the presence of other numerous beneficial compounds such as polyphenols, alkaloids, lipids, vitamins and minerals have been reported. The aim of this study was to individuate and determinate the principal bioactive compounds occurring in different part (leaves, buds and flowers) of wild and cultivated C. spinosa collected from different area of Sardinia (Italy). Ultra-high performance liquid chromatography-triple quadrupole/linear ion trap tandem mass spectrometry methods were used for identification and simultaneous determination of 27 bioactive molecules. Analysis of different samples revealed qualitative and quantitative differences in the content of flavonoids, glucosinolates, anthocyanins and phenolic acids. In particular, glucocapparin resulted the most abundant with values ranging from 112 to 364 mg/100 g Fresh Weight (FW); followed by rutin with highest value of 126 mg/100 g FW, 4-Hydroxyglucobrassicin with highest value of 42 mg/100 g FW and isorhamnetin 3-O-rutinoside with highest value of 24 mg/100 g FW. Based on this metabolomic targeted approach, quantitative results were treated by principal component analysis to explore and visualise correlation and discrimination among collections of C. spinosa samples. Copyright (c) 2016 John Wiley & Sons, Ltd.


