WAY 161503 hydrochloridePotent, selective 5-HT2C agonist CAS# 276695-22-8 |
- Granisetron HCl
Catalog No.:BCC1060
CAS No.:107007-99-8
- SB 271046 hydrochloride
Catalog No.:BCC1924
CAS No.:209481-24-3
- Adoprazine
Catalog No.:BCC1329
CAS No.:222551-17-9
- SEA0400
Catalog No.:BCC1941
CAS No.:223104-29-8
- Tianeptine
Catalog No.:BCC1999
CAS No.:66981-73-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 276695-22-8 | SDF | Download SDF |
| PubChem ID | 21976692 | Appearance | Powder |
| Formula | C11H12Cl3N3O | M.Wt | 308.59 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
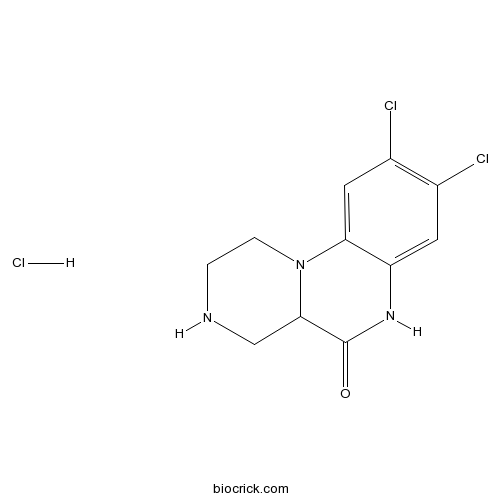
| Chemical Name | 8,9-dichloro-1,2,3,4,4a,6-hexahydropyrazino[1,2-a]quinoxalin-5-one;hydrochloride | ||
| SMILES | C1CN2C(CN1)C(=O)NC3=CC(=C(C=C32)Cl)Cl.Cl | ||
| Standard InChIKey | YPNWSZJDAKOUAW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H11Cl2N3O.ClH/c12-6-3-8-9(4-7(6)13)16-2-1-14-5-10(16)11(17)15-8;/h3-4,10,14H,1-2,5H2,(H,15,17);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective 5-HT2C receptor agonist (Ki = 4 nM; EC50 = 12 nM). Antidepressant following systemic administration in vivo. |

WAY 161503 hydrochloride Dilution Calculator

WAY 161503 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2405 mL | 16.2027 mL | 32.4055 mL | 64.8109 mL | 81.0136 mL |
| 5 mM | 0.6481 mL | 3.2405 mL | 6.4811 mL | 12.9622 mL | 16.2027 mL |
| 10 mM | 0.3241 mL | 1.6203 mL | 3.2405 mL | 6.4811 mL | 8.1014 mL |
| 50 mM | 0.0648 mL | 0.3241 mL | 0.6481 mL | 1.2962 mL | 1.6203 mL |
| 100 mM | 0.0324 mL | 0.162 mL | 0.3241 mL | 0.6481 mL | 0.8101 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Akt/SKG Substrate Peptide
Catalog No.:BCC5748
CAS No.:276680-69-4
- Leucoside
Catalog No.:BCN5167
CAS No.:27661-51-4
- Cyanidin-3-O-galactoside chloride
Catalog No.:BCN3022
CAS No.:27661-36-5
- Boc-Ser-OMe
Catalog No.:BCC3441
CAS No.:2766-43-0
- 5-Isoquinolinesulfonic acid
Catalog No.:BCC8749
CAS No.:27655-40-9
- 15-Nonacosanol
Catalog No.:BCC8439
CAS No.:2764-81-0
- Muscimol
Catalog No.:BCC6593
CAS No.:2763-96-4
- Rhodexin B
Catalog No.:BCC8246
CAS No.:2763-20-4
- Danaidal
Catalog No.:BCN1965
CAS No.:27628-46-2
- Communic acid
Catalog No.:BCN5166
CAS No.:2761-77-5
- MMF
Catalog No.:BCC7941
CAS No.:2756-87-8
- Baccatin III
Catalog No.:BCN5165
CAS No.:27548-93-2
- Cryptomoscatone D2
Catalog No.:BCN7203
CAS No.:276856-55-4
- 7-Megastigmene-3,5,6,9-tetraol
Catalog No.:BCN5168
CAS No.:276870-26-9
- 2,4-Bis(α,α-dimethylbenzyl)phenol
Catalog No.:BCC8498
CAS No.:2772-45-4
- Scutellarin
Catalog No.:BCN5902
CAS No.:27740-01-8
- Erysotrine
Catalog No.:BCN5170
CAS No.:27740-43-8
- Geniposidic acid
Catalog No.:BCN5171
CAS No.:27741-01-1
- Pahutoxin
Catalog No.:BCN1811
CAS No.:27742-14-9
- 5-Hydroxy-1-methoxyxanthone
Catalog No.:BCN6575
CAS No.:27770-13-4
- Ervamycine
Catalog No.:BCN5172
CAS No.:27773-39-3
- 9-Angeloylretronecine N-oxide
Catalog No.:BCN2037
CAS No.:27773-86-0
- Uncarinic acid E
Catalog No.:BCN6774
CAS No.:277751-61-8
- Loroquine
Catalog No.:BCN2008
CAS No.:27792-82-1
Design, synthesis, and pharmacological characterization of N- and O-substituted 5,6,7,8-tetrahydro-4H-isoxazolo[4,5-d]azepin-3-ol analogues: novel 5-HT(2A)/5-HT(2C) receptor agonists with pro-cognitive properties.[Pubmed:23301527]
J Med Chem. 2013 Feb 14;56(3):1211-27.
The isoxazol-3-one tautomer of the bicyclic isoxazole, 5,6,7,8-tetrahydro-4H-isoxazolo[4,5-d]azepin-3-ol (THAZ), has previously been shown to be a weak GABA(A) and glycine receptor antagonist. In the present study, the potential in this scaffold has been explored through the synthesis and pharmacological characterization of a series of N- and O-substituted THAZ analogues. The analogues N-Bn-THAZ (3d) and O-Bn-THAZ (4d) were found to be potent agonists of the human 5-HT(2A) and 5-HT(2C) receptors. Judging from an elaborate pharmacological profiling at numerous other CNS targets, the 3d analogue appears to be selective for the two receptors. Administration of 3d substantially improved the cognitive performance of mice in a place recognition Y-maze model, an effect fully reversible by coadministration of the selective 5-HT(2C) antagonist SB242084. In conclusion, as novel bioavailable cognitive enhancers that most likely mediate their effects through 5-HT(2A) and/or 5-HT(2C) receptors, the isoxazoles 3d and 4d constitute interesting leads for further medicinal chemistry development.
Antidepressant-like behavioral effects mediated by 5-Hydroxytryptamine(2C) receptors.[Pubmed:11082448]
J Pharmacol Exp Ther. 2000 Dec;295(3):1120-6.
The role of the 5-HT(2C) receptor in mediating active behaviors in the modified rat forced swim test was examined. Three novel selective 5-HT(2C) receptor agonists, WAY 161503 (0.1-3.0 mg/kg), RO 60-0175 (2-20 mg/kg), and RO 60-0332 (20 mg/kg), all decreased immobility and increased swimming, a pattern of behavior similar to that which occurs with the selective serotonin reuptake inhibitor fluoxetine (5-20 mg/kg). However, the prototypical but nonselective 5-HT(2C) receptor agonist m-chlorophenylpiperazine (1-10 mg/kg) increased immobility scores in the forced swim test. The selective 5-HT(2C) receptor antagonist SB 206533 was inactive when given alone (1-20 mg/kg). However, SB 206533 (20 mg/kg) blocked the antidepressant-like effects of both WAY 161503 (1 mg/kg) and fluoxetine (20 mg/kg). The atypical antidepressant (noradrenergic alpha(2) and 5-HT(2C) receptor antagonist) mianserin reduced immobility and increased climbing at 30 mg/kg. At a behaviorally subactive dose (10 mg/kg), mianserin abolished the effects of WAY 161503 (1 mg/kg) on both swimming and immobility scores. Mianserin blocked the effects of fluoxetine (20 mg/kg) on swimming only; mianserin plus fluoxetine reduced immobility and induced a switch to climbing behavior, suggesting activation of noradrenergic transmission. These data exemplify the benefits of using the modified rat forced swim test, which was sensitive to serotonergic compounds and distinguished behavioral changes associated with serotonergic and noradrenergic effects. Taken together, the results strongly implicate a role for 5-HT(2C) receptors in the behavioral effects of antidepressant drugs.
Synthesis and 5-hydroxytryptamine (5-HT) activity of 2,3,4,4a-tetrahydro-1H-pyrazino[1,2-a]quinoxalin-5-(6H)ones and 2,3,4,4a,5,6-hexahydro-1H-pyrazino[1,2-a]quinoxalines.[Pubmed:10987434]
Bioorg Med Chem Lett. 2000 Sep 4;10(17):1991-4.
A series of 2,3,4,4a-tetrahydro-1H-pyrazino[1,2-a]quinoxalin-5-(6H)ones and 2,3,4,4a,5,6-hexahydro-1H-pyrazino[1,2-a]quinoxalines was shown to exhibit 5-HT2C agonist binding and functional activity. Compound 21R inhibited food intake over 2 h in fasted, male Sprague Dawley rats with ED50 values of 2 mg/kg (i.p.) and 10 mg/kg (p.o.).


