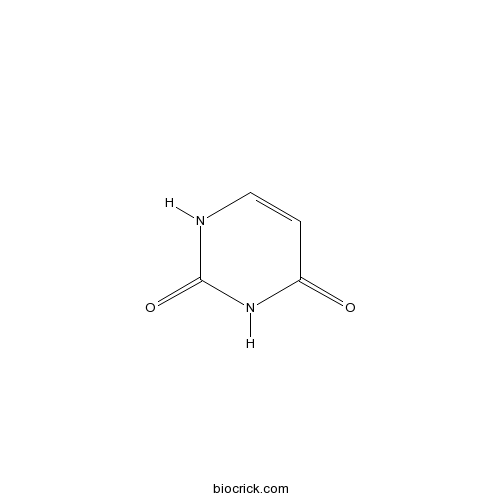
UracilCAS# 66-22-8 |
- MK-4827 tosylate
Catalog No.:BCC4174
CAS No.:1038915-73-9
- MK-4827 Racemate
Catalog No.:BCC5179
CAS No.:1038915-75-1
- BYK 204165
Catalog No.:BCC2449
CAS No.:1104546-89-5
- EB 47
Catalog No.:BCC2452
CAS No.:1190332-25-2
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- A-966492
Catalog No.:BCC2211
CAS No.:934162-61-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 66-22-8 | SDF | Download SDF |
| PubChem ID | 1174 | Appearance | Powder |
| Formula | C4H4N2O2 | M.Wt | 112.1 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
| Chemical Name | 1H-pyrimidine-2,4-dione | ||
| SMILES | C1=CNC(=O)NC1=O | ||
| Standard InChIKey | ISAKRJDGNUQOIC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C4H4N2O2/c7-3-1-2-5-4(8)6-3/h1-2H,(H2,5,6,7,8) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Uracil is a common and naturally occurring pyrimidine derivative and one of the four nucleobases in the nucleic acid of RNA, it can be used for drug delivery and as a pharmaceutical. |
| Targets | DNA/RNA Synthesis |
| In vitro | Defective repair of uracil causes telomere defects in mouse hematopoietic cells.[Pubmed: 25572391]J Biol Chem. 2015 Feb 27;290(9):5502-11.Uracil in the genome can result from misincorporation of dUTP instead of dTTP during DNA synthesis, and is primarily removed by Uracil DNA glycosylase (UNG) during base excision repair. Telomeres contain long arrays of TTAGGG repeats and may be susceptible to Uracil misincorporation. |
| Structure Identification | J Biol Chem. 2014 Aug 8;289(32):22008-18.Excision of uracil from transcribed DNA negatively affects gene expression.[Pubmed: 24951587]Uracil is an unavoidable aberrant base in DNA, the repair of which takes place by a highly efficient base excision repair mechanism. The removal of Uracil from the genome requires a succession of intermediate products, including an abasic site and a single strand break, before the original DNA structure can be reconstituted. These repair intermediates are harmful for DNA replication and also interfere with transcription under cell-free conditions. However, their relevance for cellular transcription has not been proved. Phys Chem Chem Phys. 2014 Sep 7;16(33):17835-44.Anionic derivatives of uracil: fragmentation and reactivity.[Pubmed: 25036757]Uracil is an essential biomolecule for terrestrial life, yet its prebiotic formation mechanisms have proven elusive for decades. Meteorites have been shown to contain Uracil and the interstellar abundance of aromatic species and nitrogen-containing molecules is well established, providing support for Uracil's presence in the interstellar medium (ISM). The ion chemistry of Uracil may provide clues to its prebiotic synthesis and role in the origin of life. The fragmentation of biomolecules provides valuable insights into their formation. Previous research focused primarily on the fragmentation and reactivity of cations derived from Uracil. |

Uracil Dilution Calculator

Uracil Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.9206 mL | 44.603 mL | 89.2061 mL | 178.4121 mL | 223.0152 mL |
| 5 mM | 1.7841 mL | 8.9206 mL | 17.8412 mL | 35.6824 mL | 44.603 mL |
| 10 mM | 0.8921 mL | 4.4603 mL | 8.9206 mL | 17.8412 mL | 22.3015 mL |
| 50 mM | 0.1784 mL | 0.8921 mL | 1.7841 mL | 3.5682 mL | 4.4603 mL |
| 100 mM | 0.0892 mL | 0.446 mL | 0.8921 mL | 1.7841 mL | 2.2302 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Uracil is one of the four nucleobases in the nucleic acid of RNA can be used for drug delivery and as a pharmaceutical.
- Punicalin
Catalog No.:BCN4961
CAS No.:65995-64-4
- Punicalagin
Catalog No.:BCN1037
CAS No.:65995-63-3
- Isogarciniaxanthone E
Catalog No.:BCN4205
CAS No.:659747-28-1
- Micranoic acid A
Catalog No.:BCN4204
CAS No.:659738-08-6
- AMG-517
Catalog No.:BCC1052
CAS No.:659730-32-2
- Esculentoside C
Catalog No.:BCN5012
CAS No.:65931-92-2
- Dienogest
Catalog No.:BCC4489
CAS No.:65928-58-7
- Danshenxinkun C
Catalog No.:BCN2471
CAS No.:65907-77-9
- Danshenxinkun B
Catalog No.:BCN2470
CAS No.:65907-76-8
- Danshenxinkun A
Catalog No.:BCN2469
CAS No.:65907-75-7
- Betaxolol
Catalog No.:BCC4342
CAS No.:659-18-7
- Tioconazole
Catalog No.:BCC4867
CAS No.:65899-73-2
- Strophantidin
Catalog No.:BCC8255
CAS No.:66-28-4
- Dicoumarol
Catalog No.:BCC9225
CAS No.:66-76-2
- Cycloheximide
Catalog No.:BCC3653
CAS No.:66-81-9
- D-Glucosamine hydrochloride
Catalog No.:BCN5982
CAS No.:66-84-2
- Psoralen
Catalog No.:BCN4219
CAS No.:66-97-7
- H-Nva-OH
Catalog No.:BCC2643
CAS No.:6600-40-4
- Cirsimaritin
Catalog No.:BCN4206
CAS No.:6601-62-3
- 6-Demethoxytangeretin
Catalog No.:BCN3844
CAS No.:6601-66-7
- (5Z)-7-Oxozeaenol
Catalog No.:BCC7724
CAS No.:66018-38-0
- H-D-Arg(NO2)-OH
Catalog No.:BCC2871
CAS No.:66036-77-9
- Glycyrin
Catalog No.:BCN7681
CAS No.:66056-18-6
- Licoisoflavone A
Catalog No.:BCN2929
CAS No.:66056-19-7
Anionic derivatives of uracil: fragmentation and reactivity.[Pubmed:25036757]
Phys Chem Chem Phys. 2014 Sep 7;16(33):17835-44.
Uracil is an essential biomolecule for terrestrial life, yet its prebiotic formation mechanisms have proven elusive for decades. Meteorites have been shown to contain Uracil and the interstellar abundance of aromatic species and nitrogen-containing molecules is well established, providing support for Uracil's presence in the interstellar medium (ISM). The ion chemistry of Uracil may provide clues to its prebiotic synthesis and role in the origin of life. The fragmentation of biomolecules provides valuable insights into their formation. Previous research focused primarily on the fragmentation and reactivity of cations derived from Uracil. In this study, we explore deprotonated Uracil-5-carboxylic acid and its anionic fragments to elucidate novel reagents of Uracil formation and to characterize the reactivity of Uracil's anionic derivatives. The structures of these fragments are identified through theoretical calculations, further fragmentation, experimental acidity bracketing, and reactivity with several detected and potential interstellar species (SO2, OCS, CS2, NO, N2O, CO, NH3, O2, and C2H4). Fragmentation is achieved through collision induced dissociation (CID) in a commercial ion trap mass spectrometer, and all reaction rate constants are measured using a modification of this instrument. Experimental data are supported by theoretical calculations at the B3LYP/6-311++G(d,p) level of theory. Lastly, the astrochemical implications of the observed fragmentation and reaction processes are discussed.
Excision of uracil from transcribed DNA negatively affects gene expression.[Pubmed:24951587]
J Biol Chem. 2014 Aug 8;289(32):22008-18.
Uracil is an unavoidable aberrant base in DNA, the repair of which takes place by a highly efficient base excision repair mechanism. The removal of Uracil from the genome requires a succession of intermediate products, including an abasic site and a single strand break, before the original DNA structure can be reconstituted. These repair intermediates are harmful for DNA replication and also interfere with transcription under cell-free conditions. However, their relevance for cellular transcription has not been proved. Here we investigated the influence of Uracil incorporated into a reporter vector on gene expression in human cells. The expression constructs contained a single Uracil opposite an adenine (to mimic dUTP misincorporation during DNA synthesis) or a guanine (imitating a product of spontaneous cytosine deamination). We found no evidence for a direct transcription arrest by Uracil in either of the two settings because the vectors containing the base modification exhibited unaltered levels of enhanced GFP reporter gene expression at early times after delivery to cells. However, the gene expression showed a progressive decline during subsequent hours. In the case of U:A pairs, this effect was retarded significantly by knockdown of UNG1/2 but not by knockdown of SMUG1 or thymine-DNA glycosylase Uracil-DNA glycosylases, proving that it is base excision by UNG1/2 that perturbs transcription of the affected gene. By contrast, the decline of expression of the U:G constructs was not influenced by either UNG1/2, SMUG1, or thymine-DNA glycosylase knockdown, strongly suggesting that there are substantial mechanistic or kinetic differences between the processing of U:A and U:G lesions in cells.
Defective repair of uracil causes telomere defects in mouse hematopoietic cells.[Pubmed:25572391]
J Biol Chem. 2015 Feb 27;290(9):5502-11.
Uracil in the genome can result from misincorporation of dUTP instead of dTTP during DNA synthesis, and is primarily removed by Uracil DNA glycosylase (UNG) during base excision repair. Telomeres contain long arrays of TTAGGG repeats and may be susceptible to Uracil misincorporation. Using model telomeric DNA substrates, we showed that the position and number of Uracil substitutions of thymine in telomeric DNA decreased recognition by the telomere single-strand binding protein, POT1. In primary mouse hematopoietic cells, Uracil was detectable at telomeres, and UNG deficiency further increased Uracil loads and led to abnormal telomere lengthening. In UNG-deficient cells, the frequencies of sister chromatid exchange and fragility in telomeres also significantly increased in the absence of telomerase. Thus, accumulation of Uracil and/or UNG deficiency interferes with telomere maintenance, thereby underscoring the necessity of UNG-initiated base excision repair for the preservation of telomere integrity.


