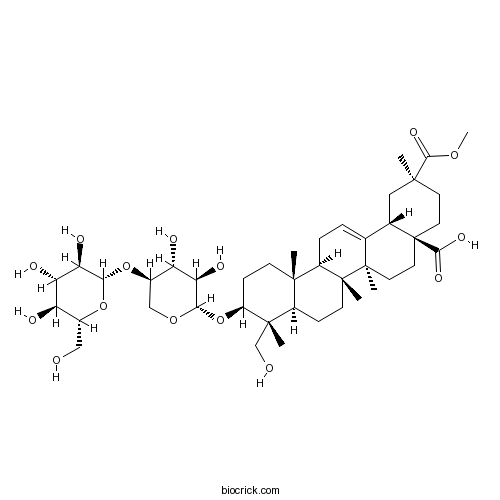
Esculentoside CCAS# 65931-92-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 65931-92-2 | SDF | Download SDF |
| PubChem ID | 10724099 | Appearance | White powder |
| Formula | C42H66O15 | M.Wt | 810.97 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,4aR,6aR,6aS,6bR,8aR,9R,10S,12aR,14bS)-10-[(2S,3R,4R,5R)-3,4-dihydroxy-5-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxan-2-yl]oxy-9-(hydroxymethyl)-2-methoxycarbonyl-2,6a,6b,9,12a-pentamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid | ||
| SMILES | CC1(CCC2(CCC3(C(=CCC4C3(CCC5C4(CCC(C5(C)CO)OC6C(C(C(CO6)OC7C(C(C(C(O7)CO)O)O)O)O)O)C)C)C2C1)C)C(=O)O)C(=O)OC | ||
| Standard InChIKey | NMDFCFOQBAHNPV-RFTJXAPBSA-N | ||
| Standard InChI | InChI=1S/C42H66O15/c1-37(36(52)53-6)13-15-42(35(50)51)16-14-40(4)21(22(42)17-37)7-8-26-38(2)11-10-27(39(3,20-44)25(38)9-12-41(26,40)5)57-33-31(48)29(46)24(19-54-33)56-34-32(49)30(47)28(45)23(18-43)55-34/h7,22-34,43-49H,8-20H2,1-6H3,(H,50,51)/t22-,23+,24+,25+,26+,27-,28+,29-,30-,31+,32+,33-,34-,37-,38-,39-,40+,41+,42-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Esculentoside C exerts proinflammatory effects synergistically, it can induce inflammatory stimulation. |
| Targets | TNF-α | IL Receptor | NO |

Esculentoside C Dilution Calculator

Esculentoside C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2331 mL | 6.1655 mL | 12.3309 mL | 24.6618 mL | 30.8273 mL |
| 5 mM | 0.2466 mL | 1.2331 mL | 2.4662 mL | 4.9324 mL | 6.1655 mL |
| 10 mM | 0.1233 mL | 0.6165 mL | 1.2331 mL | 2.4662 mL | 3.0827 mL |
| 50 mM | 0.0247 mL | 0.1233 mL | 0.2466 mL | 0.4932 mL | 0.6165 mL |
| 100 mM | 0.0123 mL | 0.0617 mL | 0.1233 mL | 0.2466 mL | 0.3083 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dienogest
Catalog No.:BCC4489
CAS No.:65928-58-7
- Danshenxinkun C
Catalog No.:BCN2471
CAS No.:65907-77-9
- Danshenxinkun B
Catalog No.:BCN2470
CAS No.:65907-76-8
- Danshenxinkun A
Catalog No.:BCN2469
CAS No.:65907-75-7
- Betaxolol
Catalog No.:BCC4342
CAS No.:659-18-7
- Tioconazole
Catalog No.:BCC4867
CAS No.:65899-73-2
- Daturabietatriene
Catalog No.:BCN4203
CAS No.:65894-41-9
- Peucedanol 3'-O-glucoside
Catalog No.:BCN7688
CAS No.:65891-61-4
- 2-(2-Aminothiazole-4-yl)-2-methoxyiminoacetic acid
Catalog No.:BCC8478
CAS No.:65872-41-5
- 3-Epiglochidiol diacetate
Catalog No.:BCN4202
CAS No.:6587-37-7
- 1-Benzyl-5-ethoxyhydantoin
Catalog No.:BCC8460
CAS No.:65855-02-9
- Z(2-Cl)-Osu
Catalog No.:BCC2691
CAS No.:65853-65-8
- AMG-517
Catalog No.:BCC1052
CAS No.:659730-32-2
- Micranoic acid A
Catalog No.:BCN4204
CAS No.:659738-08-6
- Isogarciniaxanthone E
Catalog No.:BCN4205
CAS No.:659747-28-1
- Punicalagin
Catalog No.:BCN1037
CAS No.:65995-63-3
- Punicalin
Catalog No.:BCN4961
CAS No.:65995-64-4
- Uracil
Catalog No.:BCN4211
CAS No.:66-22-8
- Strophantidin
Catalog No.:BCC8255
CAS No.:66-28-4
- Dicoumarol
Catalog No.:BCC9225
CAS No.:66-76-2
- Cycloheximide
Catalog No.:BCC3653
CAS No.:66-81-9
- D-Glucosamine hydrochloride
Catalog No.:BCN5982
CAS No.:66-84-2
- Psoralen
Catalog No.:BCN4219
CAS No.:66-97-7
- H-Nva-OH
Catalog No.:BCC2643
CAS No.:6600-40-4
Rabbit conjunctivae edema and release of NO, TNF-alpha, and IL-1beta from macrophages induced by fractions and esculentosides isolated from Phytolacca americana.[Pubmed:25894210]
Pharm Biol. 2016;54(1):98-104.
CONTEXT: The roots of Phytolacca americana L. (Phytolaccaceae) may be toxic. Despite heated controversy over the toxic compounds of P. americana, especially esculentosides, relevant studies remain scarce. OBJECTIVE: The objective of this study is to screen the toxic fractions and compounds of P. americana, to determine the controlling indices, and to provide evidence for unraveling the mechanism. MATERIALS AND METHODS: Petroleum ether (PE), CH2Cl2, n-BuOH, and water fractions were isolated from 70% ethanol extract of P. americana. The n-BuOH fraction was dissolved in 50% ethanol and precipitated by adding ethyl ether. The resultant supernatants and precipitates were referred to as SUPs and SEDs fractions, respectively. SUPs fraction was separated by column chromatography into four main stimulating esculentosides that were identified by HR-ESI/MS and NMR as EsA, EsB, EsC, and EsF. The irritating effects of esculentosides on rabbit conjunctivae (500 mug/eye) was observed by pathological examination and those on macrophages (5, 25, 50 and 100 mug/mL) were evaluated by detecting changes of NO, TNF-alpha, and IL-1beta levels. RESULTS AND DISCUSSION: n-BuOH, SUP fractions, and EsC induced severe conjunctival edema. The four esculentosides induced dose-dependent releases of proinflammatory mediators NO, TNF-alpha, and IL-1beta from macrophages, and releasing amounts peaked after 2 h of treatment. EsC and EsF induced macrophages to release mediators most significantly. EsC (50 mug/mL) functioned more effectively than EsF did, and similarly n-BuOH and SUPs fractions functioned more effectively than the esculentoside mixture. Thus, the four esculentosides exerted proinflammatory effects synergistically. CONCLUSION: All extracted esculentosides, especially EsC, induced inflammatory stimulation. Phytolacca americana-induced irritation of the gastrointestinal tract may be associated with esculentosides such as EsC.


