GlycyrinCAS# 66056-18-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 66056-18-6 | SDF | Download SDF |
| PubChem ID | 480787 | Appearance | Powder |
| Formula | C22H22O6 | M.Wt | 382.40 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
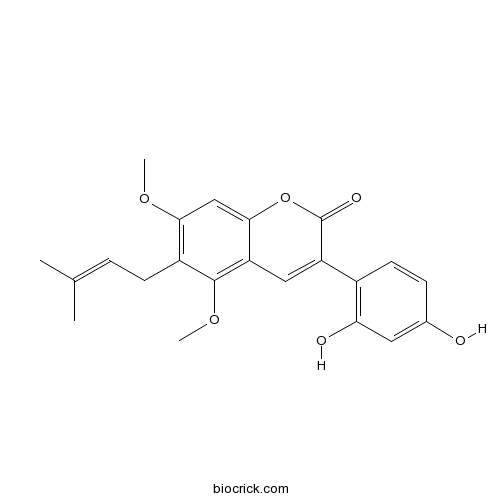
| Chemical Name | 3-(2,4-dihydroxyphenyl)-5,7-dimethoxy-6-(3-methylbut-2-enyl)chromen-2-one | ||
| SMILES | CC(=CCC1=C(C=C2C(=C1OC)C=C(C(=O)O2)C3=C(C=C(C=C3)O)O)OC)C | ||
| Standard InChIKey | FWWGXZYUURXJLK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H22O6/c1-12(2)5-7-15-19(26-3)11-20-17(21(15)27-4)10-16(22(25)28-20)14-8-6-13(23)9-18(14)24/h5-6,8-11,23-24H,7H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Glycyrin, one of the main PPAR-gamma ligands of licorice, can significantly decrease the blood glucose levels of genetically diabetic KK-A(y) mice. 2. Glycyrin exhibits anti-BsFtsZ GTPase activities, at levels comparable to that of the synthetic FtsZ inhibitor, Zantrin Z3. 3. Glycyrin has anti-hepatitis C virus (HCV) activity with the IC(50) value of 7.2 ug/mL, it would be a good candidate to develop antivirals against HCV. 4. Glycyrin possesses weaker anti-Helicobacter pylori activity, it may be a useful chemopreventive agent for peptic ulcer or gastric cancer in H. pylori-infected individuals. 5. Glycyrin shows antibacterial activity against upper airway respiratory tract bacteria such as Streptococcus pyogenes, Haemophilus influenzae and Moraxella catarrhalis. |
| Targets | HCV | PPAR | Antifection |

Glycyrin Dilution Calculator

Glycyrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6151 mL | 13.0753 mL | 26.1506 mL | 52.3013 mL | 65.3766 mL |
| 5 mM | 0.523 mL | 2.6151 mL | 5.2301 mL | 10.4603 mL | 13.0753 mL |
| 10 mM | 0.2615 mL | 1.3075 mL | 2.6151 mL | 5.2301 mL | 6.5377 mL |
| 50 mM | 0.0523 mL | 0.2615 mL | 0.523 mL | 1.046 mL | 1.3075 mL |
| 100 mM | 0.0262 mL | 0.1308 mL | 0.2615 mL | 0.523 mL | 0.6538 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-D-Arg(NO2)-OH
Catalog No.:BCC2871
CAS No.:66036-77-9
- (5Z)-7-Oxozeaenol
Catalog No.:BCC7724
CAS No.:66018-38-0
- 6-Demethoxytangeretin
Catalog No.:BCN3844
CAS No.:6601-66-7
- Cirsimaritin
Catalog No.:BCN4206
CAS No.:6601-62-3
- H-Nva-OH
Catalog No.:BCC2643
CAS No.:6600-40-4
- Psoralen
Catalog No.:BCN4219
CAS No.:66-97-7
- D-Glucosamine hydrochloride
Catalog No.:BCN5982
CAS No.:66-84-2
- Cycloheximide
Catalog No.:BCC3653
CAS No.:66-81-9
- Dicoumarol
Catalog No.:BCC9225
CAS No.:66-76-2
- Strophantidin
Catalog No.:BCC8255
CAS No.:66-28-4
- Uracil
Catalog No.:BCN4211
CAS No.:66-22-8
- Punicalin
Catalog No.:BCN4961
CAS No.:65995-64-4
- Licoisoflavone A
Catalog No.:BCN2929
CAS No.:66056-19-7
- Gomisin H
Catalog No.:BCN3902
CAS No.:66056-20-0
- Angeloylgomisin H
Catalog No.:BCN2843
CAS No.:66056-22-2
- Benzoylgomisin H
Catalog No.:BCN7242
CAS No.:66056-23-3
- Licoisoflavone B
Catalog No.:BCN6695
CAS No.:66056-30-2
- Licoisoflavanone
Catalog No.:BCN6856
CAS No.:66067-26-3
- Tigloylgomisin H
Catalog No.:BCN6927
CAS No.:66069-55-4
- GW 542573X
Catalog No.:BCC7914
CAS No.:660846-41-3
- Nimodipine
Catalog No.:BCC3823
CAS No.:66085-59-4
- GW843682X
Catalog No.:BCC1614
CAS No.:660868-91-7
- Docosanol (Abreua)
Catalog No.:BCC3769
CAS No.:661-19-8
- Glochidone
Catalog No.:BCN4207
CAS No.:6610-55-5
Filamenting temperature-sensitive mutant Z inhibitors from Glycyrrhiza glabra and their inhibitory mode of action.[Pubmed:28196701]
Bioorg Med Chem Lett. 2017 Mar 15;27(6):1420-1424.
FtsZ is an essential protein for bacterial cell division, and an attractive and underexploited novel antibacterial target protein. Screening of Indonesian plants revealed the inhibitory activity of the methanol extract of Glycyrrhiza glabra on the Bacillus subtilis FtsZ (BsFtsZ) GTPase, and further bioassay-guided fractionation of the active methanol extract led to the isolation of seven known polyketides (1-7). Among them, gancaonin I (1), Glycyrin (3), and isolicoflavanol (5) exhibited anti-BsFtsZ GTPase activities, at levels comparable to that of the synthetic FtsZ inhibitor, Zantrin Z3. Enzymatic assays using a BsFtsZ Val307R mutant protein and in silico simulations suggested that 1, 3, and 5 bind to the cleft on BsFtsZ, as in the case of the previously reported uncompetitive FtsZ inhibitor, PC190723, and thereby display their significant anti-BsFtsZ inhibitory activities. Furthermore, 1 also showed significant inhibitory activity against B. subtilis, with a MIC value of 5muM. The present study provides new insights into the naturally occurring B. subtilis growth inhibitors.
Anti-hepatitis C virus compounds obtained from Glycyrrhiza uralensis and other Glycyrrhiza species.[Pubmed:24397541]
Microbiol Immunol. 2014 Mar;58(3):180-7.
Development of complementary and/or alternative drugs for treatment of hepatitis C virus (HCV) infection is still much needed from clinical and economic points of view. Antiviral substances obtained from medicinal plants are potentially good targets to study. Glycyrrhiza uralensis and G. glabra have been commonly used in both traditional and modern medicine. In this study, extracts of G. uralensis roots and their components were examined for anti-HCV activity using an HCV cell culture system. It was found that a methanol extract of G. uralensis roots and its chloroform fraction possess anti-HCV activity with 50%-inhibitory concentrations (IC(50)) of 20.0 and 8.0 mug/mL, respectively. Through bioactivity-guided purification and structural analysis, glycycoumarin, Glycyrin, glycyrol and liquiritigenin were isolated and identified as anti-HCV compounds, their IC(50) being 8.8, 7.2, 4.6 and 16.4 mug/mL, respectively. However, glycyrrhizin, the major constituent of G. uralensis, and its monoammonium salt, showed only marginal anti-HCV activity. It was also found that licochalcone A and glabridin, known to be exclusive constituents of G. inflata and G. glabra, respectively, did have anti-HCV activity, their IC(50) being 2.5 and 6.2 mug/mL, respectively. Another chalcone, isoliquiritigenin, also showed anti-HCV activity, with an IC(50) of 3.7 mug/mL. Time-of-addition analysis revealed that all Glycyrrhiza-derived anti-HCV compounds tested in this study act at the post-entry step. In conclusion, the present results suggest that glycycoumarin, Glycyrin, glycyrol and liquiritigenin isolated from G. uralensis, as well as isoliquiritigenin, licochalcone A and glabridin, would be good candidates for seed compounds to develop antivirals against HCV.
Phenolics with PPAR-gamma ligand-binding activity obtained from licorice (Glycyrrhiza uralensis roots) and ameliorative effects of glycyrin on genetically diabetic KK-A(y) mice.[Pubmed:14643306]
Bioorg Med Chem Lett. 2003 Dec 15;13(24):4267-72.
The EtOAc extract of licorice (Glycyrrhiza uralensis roots) exhibited considerable PPAR-gamma ligand-binding activity. Bioassay-guided fractionation of the extract using a GAL-4-PPAR-gamma chimera assay method resulted in the isolation of two isoflavenes, one of which is a new compound named dehydroglyasperin D, an isoflavan, two 3-arylcoumarins, and an isoflavanone as the PPAR-gamma ligand-binding active ingredients of licorice. The isoprenyl group at C-6 and the C-2' hydroxyl group in the aromatic ring-C part in the isoflavan, isoflavene, or arylcoumarin skeleton were found to be the structural requirements for PPAR-gamma ligand-binding activity. Glycyrin, one of the main PPAR-gamma ligands of licorice, significantly decreased the blood glucose levels of genetically diabetic KK-A(y) mice.
Anti-Helicobacter pylori flavonoids from licorice extract.[Pubmed:12127165]
Life Sci. 2002 Aug 9;71(12):1449-63.
Licorice is the most used crude drug in Kampo medicines (traditional Chinese medicines modified in Japan). The extract of the medicinal plant is also used as the basis of anti-ulcer medicines for treatment of peptic ulcer. Among the chemical constituents of the plant, glabridin and glabrene (components of Glycyrrhiza glabra), licochalcone A (G. inflata), licoricidin and licoisoflavone B (G. uralensis) exhibited inhibitory activity against the growth of Helicobacter pylori in vitro. These flavonoids also showed anti-H. pylori activity against a clarithromycin (CLAR) and amoxicillin (AMOX)-resistant strain. We also investigated the methanol extract of G. uralensis. From the extract, three new isoflavonoids (3-arylcoumarin, pterocarpan, and isoflavan) with a pyran ring, gancaonols A[bond]C, were isolated together with 15 known flavonoids. Among these compounds, vestitol, licoricone, 1-methoxyphaseollidin and gancaonol C exhibited anti-H. pylori activity against the CLAR and AMOX-resistant strain as well as four CLAR (AMOX)-sensitive strains. Glycyrin, formononetin, isolicoflavonol, glyasperin D, 6,8-diprenylorobol, gancaonin I, dihydrolicoisoflavone A, and gancaonol B possessed weaker anti-H. pylori activity. These compounds may be useful chemopreventive agents for peptic ulcer or gastric cancer in H. pylori-infected individuals.
Antibacterial compounds of licorice against upper airway respiratory tract pathogens.[Pubmed:11575586]
J Nutr Sci Vitaminol (Tokyo). 2001 Jun;47(3):270-3.
The antibacterial activity of compounds obtained from licorice was measured against upper airway respiratory tract bacteria such as Streptococcus pyogenes, Haemophilus influenzae and Moraxella catarrhalis. Among the tested compounds, licoricidin exhibited the highest activity against all tested microorganisms with an MIC of 12.5 microg/mL. Three coumarin derivatives, glycyrol, Glycyrin and glycycoumarin also showed antibacterial activity.


