Tropine benzilateCAS# 3736-36-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3736-36-5 | SDF | Download SDF |
| PubChem ID | 1138166 | Appearance | Powder |
| Formula | C22H25NO3 | M.Wt | 351.45 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Tropine benzylate | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
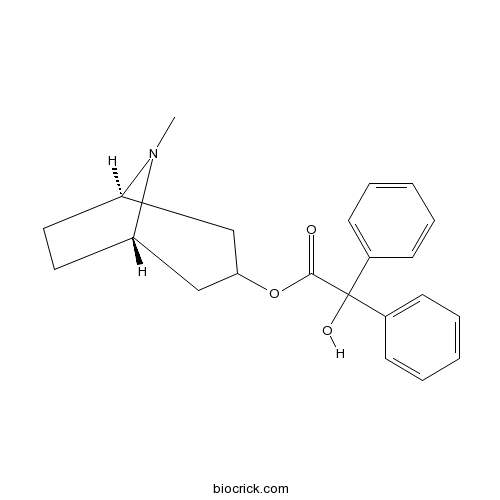
| Chemical Name | [(1R,5S)-8-methyl-8-azabicyclo[3.2.1]octan-3-yl] 2-hydroxy-2,2-diphenylacetate | ||
| SMILES | CN1C2CCC1CC(C2)OC(=O)C(C3=CC=CC=C3)(C4=CC=CC=C4)O | ||
| Standard InChIKey | XXVZGWNHSCGMCT-YOFSQIOKSA-N | ||
| Standard InChI | InChI=1S/C22H25NO3/c1-23-18-12-13-19(23)15-20(14-18)26-21(24)22(25,16-8-4-2-5-9-16)17-10-6-3-7-11-17/h2-11,18-20,25H,12-15H2,1H3/t18-,19+,20? | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tropine is a naturally occurring tropane alkaloid that serves as an intermediate in the synthesis of a variety of bioactive alkaloids, many of which have potent neurological actions. |
| Targets | NADPH |
| In vivo | Structure-activity relationships among derivatives of dicarboxylic acid esters of tropine.[Pubmed: 12441175 ]Pharmacol Ther. 2002 Oct;96(1):1-21.Several categories of neuromuscular blocking bisquaternary Tropine and tropane derivatives were synthesized and studied in the past five decades, mainly with the purpose of arriving at meaningful information about structure-activity relationships. Such a structure-activity relationship database is important in the development of new muscle relaxants with improved pharmacological characteristics.
Although quaternary Tropine diesters were explored since 1952, most of them were developed in the last decade. Over 250 such agents are being reviewed here.
|
| Kinase Assay | Tropine forming tropinone reductase gene from Withania somnifera (Ashwagandha): biochemical characteristics of the recombinant enzyme and novel physiological overtones of tissue-wide gene expression patterns.[Pubmed: 24086372]PLoS One. 2013 Sep 25;8(9):e74777.Withania somnifera is one of the most reputed medicinal plants of Indian systems of medicine synthesizing diverse types of secondary metabolites such as withanolides, alkaloids, withanamides etc. Present study comprises cloning and E. coli over-expression of a tropinone reductase gene (WsTR-I) from W. somnifera, and elucidation of biochemical characteristics and physiological role of tropinone reductase enzyme in tropane alkaloid biosynthesis in aerial tissues of the plant.
|
| Structure Identification | Biochem J. 1995 Apr 15;307 ( Pt 2):603-8.Tropine dehydrogenase: purification, some properties and an evaluation of its role in the bacterial metabolism of tropine.[Pubmed: 7733902]Tropine dehydrogenase was induced by growth of Pseudomonas AT3 on aTropine, Tropine or tropinone. It was NADP(+)-dependent and gave no activity with NAD+. The enzyme was very unstable but a rapid purification procedure using affinity chromatography that gave highly purified enzyme was developed.
|

Tropine benzilate Dilution Calculator

Tropine benzilate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8454 mL | 14.2268 mL | 28.4535 mL | 56.9071 mL | 71.1339 mL |
| 5 mM | 0.5691 mL | 2.8454 mL | 5.6907 mL | 11.3814 mL | 14.2268 mL |
| 10 mM | 0.2845 mL | 1.4227 mL | 2.8454 mL | 5.6907 mL | 7.1134 mL |
| 50 mM | 0.0569 mL | 0.2845 mL | 0.5691 mL | 1.1381 mL | 1.4227 mL |
| 100 mM | 0.0285 mL | 0.1423 mL | 0.2845 mL | 0.5691 mL | 0.7113 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Veratraldehyde
Catalog No.:BCN6089
CAS No.:120-14-9
- Scoparone
Catalog No.:BCN6088
CAS No.:120-08-1
- Sulfuretin
Catalog No.:BCN4725
CAS No.:120-05-8
- Unedone
Catalog No.:BCN6759
CAS No.:1199815-09-2
- INT-777
Catalog No.:BCC5390
CAS No.:1199796-29-6
- 1,2-Didehydrotanshinone IIA
Catalog No.:BCN3143
CAS No.:119963-50-7
- Peroxy Orange 1
Catalog No.:BCC6336
CAS No.:1199576-10-7
- 7-O-Methylmorroniside
Catalog No.:BCN7293
CAS No.:119943-46-3
- CGRP 8-37 (human)
Catalog No.:BCC5724
CAS No.:119911-68-1
- Posaconazole hydrate
Catalog No.:BCC4234
CAS No.:1198769-38-8
- Esomeprazole magnesium salt
Catalog No.:BCC5430
CAS No.:1198768-91-0
- Phyperunolide E
Catalog No.:BCN7292
CAS No.:1198400-52-0
- Clorofene
Catalog No.:BCC8919
CAS No.:120-32-1
- 3-Amino-4-methoxybenzanilide
Catalog No.:BCC8613
CAS No.:120-35-4
- Desoxyanisoin
Catalog No.:BCN2264
CAS No.:120-44-5
- Ethylparaben
Catalog No.:BCN6094
CAS No.:120-47-8
- Benzyl benzoate
Catalog No.:BCN8521
CAS No.:120-51-4
- Isosafrole
Catalog No.:BCC3976
CAS No.:120-58-1
- 2'-Methylacetanilide
Catalog No.:BCC8581
CAS No.:120-66-1
- N,N'-Bis(salicylidene)-1,3-propanediamine
Catalog No.:BCC9063
CAS No.:120-70-7
- 1,2-Benzenediol
Catalog No.:BCN6103
CAS No.:120-80-9
- Dichlorphenamide
Catalog No.:BCC3761
CAS No.:120-97-8
- Donepezil HCl
Catalog No.:BCC4569
CAS No.:120011-70-3
- VCH-916
Catalog No.:BCC2031
CAS No.:1200133-34-1


