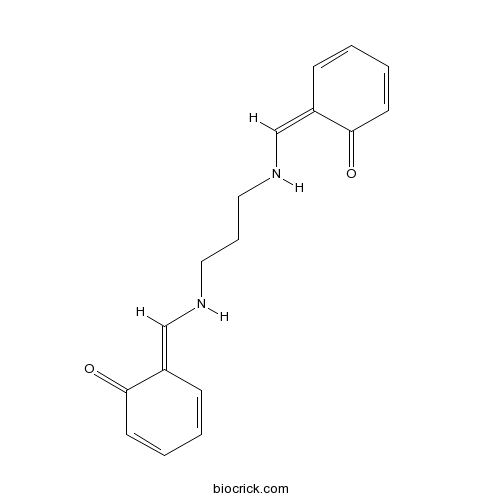
N,N'-Bis(salicylidene)-1,3-propanediamineCAS# 120-70-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 120-70-7 | SDF | Download SDF |
| PubChem ID | 5464128 | Appearance | Powder |
| Formula | C17H18N2O2 | M.Wt | 282.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (6Z)-6-[[3-[[(E)-(6-oxocyclohexa-2,4-dien-1-ylidene)methyl]amino]propylamino]methylidene]cyclohexa-2,4-dien-1-one | ||
| SMILES | C1=CC(=CNCCCNC=C2C=CC=CC2=O)C(=O)C=C1 | ||
| Standard InChIKey | BHBZPGSHCVSMEB-PMJBJKLVSA-N | ||
| Standard InChI | InChI=1S/C17H18N2O2/c20-16-8-3-1-6-14(16)12-18-10-5-11-19-13-15-7-2-4-9-17(15)21/h1-4,6-9,12-13,18-19H,5,10-11H2/b14-12-,15-13+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

N,N'-Bis(salicylidene)-1,3-propanediamine Dilution Calculator

N,N'-Bis(salicylidene)-1,3-propanediamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5423 mL | 17.7117 mL | 35.4233 mL | 70.8466 mL | 88.5583 mL |
| 5 mM | 0.7085 mL | 3.5423 mL | 7.0847 mL | 14.1693 mL | 17.7117 mL |
| 10 mM | 0.3542 mL | 1.7712 mL | 3.5423 mL | 7.0847 mL | 8.8558 mL |
| 50 mM | 0.0708 mL | 0.3542 mL | 0.7085 mL | 1.4169 mL | 1.7712 mL |
| 100 mM | 0.0354 mL | 0.1771 mL | 0.3542 mL | 0.7085 mL | 0.8856 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2'-Methylacetanilide
Catalog No.:BCC8581
CAS No.:120-66-1
- Isosafrole
Catalog No.:BCC3976
CAS No.:120-58-1
- Benzyl benzoate
Catalog No.:BCN8521
CAS No.:120-51-4
- Ethylparaben
Catalog No.:BCN6094
CAS No.:120-47-8
- Desoxyanisoin
Catalog No.:BCN2264
CAS No.:120-44-5
- 3-Amino-4-methoxybenzanilide
Catalog No.:BCC8613
CAS No.:120-35-4
- Clorofene
Catalog No.:BCC8919
CAS No.:120-32-1
- Tropine benzilate
Catalog No.:BCN1921
CAS No.:3736-36-5
- Veratraldehyde
Catalog No.:BCN6089
CAS No.:120-14-9
- Scoparone
Catalog No.:BCN6088
CAS No.:120-08-1
- Sulfuretin
Catalog No.:BCN4725
CAS No.:120-05-8
- Unedone
Catalog No.:BCN6759
CAS No.:1199815-09-2
- 1,2-Benzenediol
Catalog No.:BCN6103
CAS No.:120-80-9
- Dichlorphenamide
Catalog No.:BCC3761
CAS No.:120-97-8
- Donepezil HCl
Catalog No.:BCC4569
CAS No.:120011-70-3
- VCH-916
Catalog No.:BCC2031
CAS No.:1200133-34-1
- Edgeworin
Catalog No.:BCN6561
CAS No.:120028-43-5
- Meridinol
Catalog No.:BCN6087
CAS No.:120051-54-9
- Shizukanolide F
Catalog No.:BCN6411
CAS No.:120061-96-3
- CRF (6-33)
Catalog No.:BCC5791
CAS No.:120066-38-8
- Fmoc-D-Arg(Mtr)-OH
Catalog No.:BCC3078
CAS No.:120075-24-3
- Flavanthrin
Catalog No.:BCN3687
CAS No.:120090-80-4
- IPI-145 (INK1197)
Catalog No.:BCC1104
CAS No.:1201438-56-3
- Crotaleschenine
Catalog No.:BCN2077
CAS No.:120154-95-2
Exploring the coordinative adaptation and molecular shapes of trinuclear CuM(II) (M = Zn/Cd) complexes derived from salen type Schiff bases: structural and theoretical studies.[Pubmed:26931060]
Dalton Trans. 2016 Apr 7;45(13):5730-40.
Three new trinuclear hetero-metallic complexes [(CuL)2Zn(NCS)2] (1), [(CuL(R))2Zn(NCS)(mu1,1-NCS)] (2) and [(CuL(R))2Cd(mu1,3-NCS)2] (4) have been synthesized using [CuL] and [CuL(R)] as "metalloligands" (where H2L = N,N'-bis(salicylidene)-1,3-propanediamine and H2L(R) = N,N'-bis(2-hydroxybenzyl)-1,3-propanediamine). All three complexes are characterized by elemental analysis, spectroscopic methods and single crystal XRD. Complex 1 is an angular trinuclear species, in which two terminal four-coordinate square planar "metalloligands" [CuL] are coordinated to a central Zn(ii) through double phenoxido bridges along with two mutually cis nitrogen atoms of terminal isothiocyanate ions as is usually found in such complexes. In contrast, in complex 2, the two terminal "metalloligands" [CuL(R)] are square pyramidal, as one of the SCN(-) ions makes an unusual mu1,1-NCS bridge between copper centers while the other one coordinates to Zn(ii) through a N atom in a usual fashion making its geometry also square pyramidal. For 4 which possesses an angular trinuclear structure, in addition to double phenoxido bridges from two terminal [CuL(R)], both the SCN(-) ions are S-bonded to Cd(ii) and form a bridge (cis-mu1,3-SCN) between Cd(ii) and each of the terminal Cu(ii) ions. This structure is different from its unreduced analogue in which NCS(-) was N-terminal coordinated to Cd(ii) (3/3'). All the structures have been optimized using density functional theory (DFT) calculations. It has been found that for H2L, optimized structures like 1 and 2 differ only by 0.4 kcal mol(-1) but the H2L(R) structure 2 is more stable by 5.5 kcal mol(-1) than the structure resembling 1. For Cd(ii) complexes also, H2L optimized structures such as 3 and 4 do not differ significantly in energy (1.0 kcal mol(-1)) but the H2L(R) structure 4 is more stable than that of 3 by 4.6 kcal mol(-1). In fact, structure 4 has been found to be the most stable one among the other possible isomers of H2L(R).
Strong ferromagnetic exchange interactions in hinge-like Dy(O2Cu)2 complexes involving double oxygen bridges.[Pubmed:26381465]
Inorg Chem. 2015 Oct 5;54(19):9543-55.
Two trinuclear isomeric compounds, [{(Cu(II)(salpn))(Me(CO)Me)}2Dy(III)(NO3)3] (1) and [{Cu(II)(salpn)}2Dy(III)(H2O)(NO3)3].MeOH (2), along with one polymeric compound, {[{Cu(II)(salpn)}2Dy(III)(NO3)3bpy].MeOH.H2O}n (3), were synthesized using a metalloligand, [Cu(II)(salpn)], where H2salpn and bpy stand for N,N'-bis(salicylidene)-1,3-propanediamine and 4,4'-bipyridine, respectively. Compounds 1 and 2 were selectively prepared with two solvents: the less polar acetone led to the exclusive crystallization of 1 with a transoid trinuclear architecture, while more polar solvent methanol provided sole construction of 2 with a cisoid trinuclear architecture. Compound 3 was prepared from 1 or 2 after bpy was introduced as a bridge. The Dy and Cu ions are doubly bridged with oxygen atoms, and the core DyO2Cu skeletons are characterized by different "butterfly angles" of 140.9(1) degrees , 147.1(19) degrees , and 142.4(2) degrees for 1, 2, and 3, respectively. We have examined the molecular structures and magnetic properties of 1-3 using high-frequency electron paramagnetic resonance (HF-EPR), magnetization, and magnetic susceptibility techniques. These compounds showed slow magnetization reversal in the measurements of alternating current magnetic susceptibility. We analyzed EPR frequency-field diagrams using an effective spin-Hamiltonian including only one doublet of Dy sublevels and found that the exchange couplings are ferromagnetic in all compounds. The exchange coupling parameters JDy-Cu of 1, 2, and 3 were determined as 2.25 +/- 0.05, 1.82 +/- 0.04, and 1.79 +/- 0.04 K, respectively. These values are larger than those found in previous research using EPR analysis on [Cu(II)(L(A))(C3H6O)Dy(III)(NO3)3] (H2L(A) = N,N'-bis(3-methoxysalicylidene)-1,3-diamino-2,2-dimethylpropane) and [Dy(III)L(B)2(NO3)2{Cu(II)(CH3OH)}2](NO3)(CH3OH) (H2L(B) = 2,6-bis(acetylaceto)pyridine). The present result shows an advantage of doubly oxygen-bridged motifs to built strong ferromagnetic interactions between lanthanide and transition metal ions. We found that the exchange coupling strength is sensitive to the structural parameters such as bond angles, bond lengths, and butterfly angles. Precise determination of the exchange parameters would contribute to development of exchange-coupled 4f-3d heterometallic complexes.
Unprecedented structural variations in trinuclear mixed valence Co(II/III) complexes: theoretical studies, pnicogen bonding interactions and catecholase-like activities.[Pubmed:25611163]
Dalton Trans. 2015 Feb 28;44(8):3862-76.
Three new mixed valence trinuclear Co(II/III) compounds cis-[Co3L2(MeOH)2(N3)2(mu1,1-N3)2] (1), trans-[Co3L2(H2O)2(N3)2(mu1,1-N3)2].(H2O)2 (2) and [Co3L(R)2(N3)3(mu1,3-N3)] (3) have been synthesized by reacting a di-Schiff base ligand (H2L) or its reduced form [H2LR] (where H2L= N,N'-bis(salicylidene)-1,3-propanediamine and H2LR= N,N'-bis(2-hydroxybenzyl)-1,3-propanediamine) with cobalt perchlorate hexahydrate and sodium azide. All three products have been characterized by IR, UV-Vis and EPR spectroscopies, ESI-MS, elemental, powder and single crystal X-ray diffraction analyses. Complex 2 is an angular trinuclear species in which two terminal octahedral Co(III)N2O4 centers coordinate to the central octahedral cobalt(II) ion through mu2-phenoxido oxygen and mu1,1-azido nitrogen atoms along with two mutually cis-oxygen atoms of methanol molecules. On the other hand, in linear trinuclear complex , in addition to the mu2-phenoxido and mu1,1-azido bridges with terminal octahedral Co(III) centres, the central Co(II) is bonded with two mutually trans-oxygen atoms of water molecules. Thus the cis-trans configuration of the central Co(II) is solvent dependent. In complex 3, the two terminal octahedral Co(III)N2O4 centers coordinate to the central penta-coordinated Co(II) ion through double phenoxido bridges along with the nitrogen atom of a terminal azido ligand. In addition, the two terminal Co(III) are connected through a mu1,3-azido bridge that participates in pnicogen bonding interactions (intermolecular N-N interaction) as an acceptor. Both the cis and trans isomeric forms of 1 and 2 have been optimized using density functional theory (DFT) calculations and it is found that the cis configuration is energetically more favorable than the trans one. However, the trans configuration of 2 is stabilized by the hydrogen bonding network involving a water dimer. The pnicogen bonding interactions have been demonstrated using MEP surfaces and CSD search which support the counter intuitive electron acceptor ability of the mu1,3-azido ligand. Complexes 1-3 exhibit catecholase-like activities in the aerial oxidation of 3,5-di-tert-butylcatechol to the corresponding o-quinone. Kinetic data analyses of this oxidation reaction in acetonitrile reveal that the catecholase-like activity follows the order: 1 (kcat = 142 h(-1)) > 3 (kcat = 99 h(-1)) > 2 (kcat = 85 h(-1)). Mechanistic investigations of the catalytic behaviors by X-band EPR spectroscopy and estimation of hydrogen peroxide formation indicate that the oxidation reaction proceeds through the reduction of Co(III) to Co(II).
Influence of the central metal ion in controlling the self-assembly and magnetic properties of 2D coordination polymers derived from [(NiL)2M](2+) nodes (M = Ni, Zn and Cd) (H2L = salen-type di-Schiff base) and dicyanamide spacers.[Pubmed:25418864]
Dalton Trans. 2015 Jan 21;44(3):1292-302.
Three new 2D coordination polymers (CPs) (2)infinity[(NiL)2Ni(mu1,5-N(CN)2)2]n (), (2)infinity[(NiL)2Cd(mu1,5-N(CN)2)2]n () and (2)infinity[(NiL)2Zn(mu1,5-N(CN)2)2]n () have been synthesized by reacting a [NiL] "metalloligand" (where H2L = N,N'-bis(salicylidene)-1,3-propanediamine) with three different metal(ii) (Ni, Cd and Zn) perchlorates and sodium dicyanamide, with identical molar ratios of the reactants. All three products have been characterized by IR and UV-Vis spectroscopies, elemental analyses, powder and single crystal X-ray diffraction and variable temperature magnetic measurements. The isomorphous compounds and consist of similar [(NiL)2M(mu1,5-N(CN)2)] (M = Ni for and Cd for ) angular trinuclear units in which two terminal "metalloligands" [NiL] coordinate to the central nickel(ii) (in ) or cadmium(ii) (in ) ion through phenoxido oxygen atoms. The mu1,5-bridging dicyanamido spacers connect the central Ni(ii) or Cd(ii) of one node to terminal Ni(ii) of two different nodes giving rise to 2D CPs. Compound also contains trinuclear units with the same formula as those of and : [(NiL)2M(mu1,5-N(CN)2)] (M = Zn in ). The main differences are that these units are linear in and the dicyanamide spacers link only the nickel atoms of neighbouring nodes. As in and , these trinuclear units are connected to four other units via four mu1,5-bridging dicyanamido ligands, giving rise to 2D CP with a similar topology: a uninodal 4-connected underlying net with the sql (Shubnikov tetragonal plane net) topology and (4(4).6(2)) point symbol. The magnetic properties show the presence of moderate intra-trimer antiferromagnetic interactions in (J = -12.9 cm(-1)) and weak antiferromagnetic interactions between the terminal Ni(ii) ions in (J = -2.4 cm(-1)). In the Ni(ii) ions are well isolated by the central Zn(ii) ion and accordingly, only a very weak antiferromagnetic interaction through the single mu1,5-bridging dicyanamido ligands is observed (J = -0.44 cm(-1), D = -3.9 cm(-1)).
Ferro- to antiferromagnetic crossover angle in diphenoxido- and carboxylato-bridged trinuclear Ni(II)(2)-Mn(II) complexes: experimental observations and theoretical rationalization.[Pubmed:25111338]
Inorg Chem. 2014 Sep 2;53(17):9296-305.
Three new trinuclear heterometallic Ni(II)-Mn(II) complexes have been synthesized using a [NiL] metalloligand, where H2L = N,N'-bis(salicylidene)-1,3-propanediamine. The complexes [(NiL)2Mn(OCnn)2(CH3OH)2].CH3OH (1), [(NiL)2Mn(OPh)2(CH3OH)2][(NiL)2Mn(OPh)2].H2O (2), and [(NiL)2Mn(OSal)2(CH3OH)2].2[NiL] (3) (where OCnn = cinnamate, OPh = phenylacetate, OSal = salicylate) have been structurally characterized. In all three complexes, in addition to the double phenoxido bridge, the two terminal Ni(II) atoms are linked to the central Mn(II) by means of a syn-syn bridging carboxylate, giving rise to a linear structure. Complexes 1 and 2 with Ni-O-Mn angles of 97.24 and 96.43 degrees , respectively, exhibit ferromagnetic interactions (J(Ni-Mn) = +1.38 and +0.50 cm(-1), respectively), whereas 3 is antiferromagnetic (J(Ni-Mn) = -0.24 cm(-1)), having an Ni-O-Mn angle of 98.51 degrees . DFT calculations indicate that there is a clear magneto-structural correlation between the Ni-O-Mn angle and J(Ni-Mn) values, which is in agreement with the experimental results.
Supramolecular 2D/3D isomerism in a compound containing heterometallic Cu(II)2Co(II) nodes and dicyanamide bridges.[Pubmed:24552535]
Inorg Chem. 2014 Mar 3;53(5):2441-9.
Three new heterometallic copper(II)-cobalt(II) complexes [(CuL(2))2Co{dca}2].H2O(1), [(CuL(1))2Co{dca}2]n (2a), and [(CuL(1))2Co{dca}2]n (2b) [dca(-) = dicyanamide = N(CN)2(-)] have been synthesized by reacting the "metallo-ligand" [CuL(1)] or [CuL(2)] with cobalt(II) perchlorate and sodium dicyanamide in methanol-water medium (where H2L(1) = N,N'-bis(salicylidene)-1,3-propanediamine and H2L(2) = N,N'-bis(alpha-methylsalicylidene)-1,3-propanediamine). The three complexes have been structurally and magnetically characterized. Complex 1 is a discrete trinuclear species in which two metallo-ligands coordinate to a cobalt(II) ion through the phenoxido oxygen atoms along with two terminally coordinated dicyanamide ions. On the other hand, complexes 2a and 2b are one of the very scarce examples of supramolecular isomers since they present the same [(CuL(1))2Co{dca}2] trinuclear units (very similar to the trinuclear core in 1) and differ only in their superstructures. Thus, although each Cu2Co trimer in 2a and 2b is connected to four other Cu2Co trimers through four mu1,5-dca(-) bridges, 2a presents a square two-dimensional structure (each Cu2Co trimer is connected to four in-plane Cu2Co trimers); whereas, 2b shows a triangular three-dimensional lattice (each Cu2Co trimer is connected to three in-plane and one out-of-plane trimers). Variable-temperature magnetic susceptibility measurements show the presence of moderate antiferromagnetic exchange interactions (ferrimagnetic) in all the cases mediated through the double phenoxido bridges that have been fitted with an anisotropic model including spin-orbit coupling in the central Co(II) ion.
Isolation of two different Ni2Zn complexes with an unprecedented cocrystal formed by one of them and a "coordination positional isomer" of the other.[Pubmed:24350719]
Inorg Chem. 2014 Jan 6;53(1):434-45.
A new homometallic trinuclear Ni(II) complex [(NiL)2Ni(NCS)2] (1) and three heterometallic trinuclear Ni(II)-Zn(II)-Ni(II) complexes [(NiL)2Zn(NCS)2] (2), [(NiL)2Zn(NCS)2(CH3OH)2].2CH3OH (3) and {[(NiL)2Zn(NCS)2(CH3OH)2]} {[(NiL)2Zn(NCS)2]} (4) have been synthesized by using the "complex as ligand" approach with the "metalloligand" [NiL] (H2L = N,N'-bis(salicylidene)-1,3-propanediamine) and thiocyanate in different ratios. All the complexes have been structurally and magnetically characterized. In the isomorphous complexes 1 and 2, the two terminal square planar Ni atoms and the central octahedral nickel atom (in 1) or zinc atom (in 2) are arranged in a bent structure where two cis kappaN-SCN(-) thiocyanate ions are coordinated to the central atom. The chemical composition of 3 is very similar to that of 2 but, in 3, the central Zn atom is tetrahedral and the kappaN-SCN(-) thiocyanate ions occupy an axial position of each terminal nickel atom (which now are octahedral with the sixth position occupied by a methanol molecule). Complex 4 consists of two closely related trinuclear units 4A and 4B. In 4A, the coordination environments of the metals are identical to those of 3 whereas 4B is a "coordination position isomer" of complex 2 with the central square pyramidal Zn and one of the terminal square pyramidal Ni atoms coordinated by two kappaN-SCN(-) thiocyanate ions. Complex 4 is a unique example of a cocrystal formed by two similar trinuclear units (4A and 4B) where 4A is identical to an existing complex (3) and 4B is a "coordination position isomer" of another existing complex (2).
Trinuclear heterometallic Cu(II)-Mn(II) complexes of a salen type Schiff base ligand: anion dependent variation of phenoxido bridging angles and magnetic coupling.[Pubmed:24162065]
Dalton Trans. 2014 Jan 21;43(3):990-8.
Five new trinuclear heterometallic Cu(II)-Mn(II) complexes [(CuL)2Mn(O2CPh)2] (1), [(CuL)2Mn(N3)2] (2), [(CuL)2Mn(NCO)2] (3), [(CuL)2Mn(NO3)2] (4) and [(CuL)2Mn(Sal)2].CH2Cl2 (5) have been synthesized with the di-Schiff base ligand H2L (where H2L = N,N'-bis(salicylidene)-1,3-propanediamine and Sal = salicylate). These complexes with different anionic co-ligands have been synthesized to attain a large variation in phenoxido bridging angles and to investigate its consequence on magnetic properties. Single crystal X-ray diffraction analyses reveal that complexes 1, 2, 4 and 5 are linear, whereas 3 has an angular geometry. Variable temperature magnetic susceptibility measurements suggest that all five complexes possess an overall antiferromagnetic interaction between Cu(II) and Mn(II) ions, which results in a final ferrimagnetic ground state with spin 3/2 in the Cu(II)-Mn(II)-Cu(II) trinuclear structure. The weakest antiferromagnetic interaction (J(Cu-Mn) = -7.0 cm(-1)) is observed for 2 having the lowest value of the Cu-O-Mn angle (92.0 degrees ), while the strongest antiferromagnetic interaction (J(Cu-Mn) = -26.5 cm(-1)) is observed for 3 having the largest Cu-O-Mn angle (101.4 degrees ). Complexes 1, 4 and 5 show average Cu-O-Mn angles of 98.2 degrees , 97.6 degrees and 97.7 degrees , respectively, that lead to intermediate antiferromagnetic interactions (J(Cu-Mn) = -9.6, -9.7, -9.3 cm(-1) respectively).
Solvent-templated supramolecular isomerism in 2D coordination polymer constructed by Ni(II)2Co(II) nodes and dicyanamido spacers: drastic change in magnetic behaviours.[Pubmed:23900267]
Dalton Trans. 2013 Oct 7;42(37):13554-64.
Two heterometallic coordination polymers (CPs) have been prepared using [Ni(II)L]2Co(II) (where H2L = N,N'-bis(salicylidene)-1,3-propanediamine) as nodes and dicyanamido spacers by varying the solvent for synthesis. Structural characterizations revealed that methanol assisted the formation of a two-dimensional (4,4) connected rhombic grid network of [(NiL)2Co(NCNCN)2]infinity (1a) whereas relatively less polar acetonitrile afforded a different superstructure {[(NiL)2Co(NCNCN)2].CH3CN}infinity (1b) with a two-dimensional (4,4) connected square grid network. The presence of acetonitrile molecules in the structure of 1b seems to change the spatial orientation of the terminal metalloligands [NiL] from pseudo-eclipsed in 1a to staggered-like in 1b around the central Co(II). These structural changes in the nodes together with the conformationally flexible dicyanamido spacers, which are cis coordinated to the Co(II) in both trinuclear units, led to the differences in the final 2D network. Variable-temperature magnetic susceptibility measurements revealed that this supramolecular isomerism led to a drastic transition from spin-frustrated antiferromagnetism for 1a to a dominant ferromagnetic behaviour for 1b. The geometrical differences in Ni2Co coordination clusters (CCs) which are scalene triangular in 1a but nearly linear in 1b, are held responsible for the changes of the magnetic properties. The DFT calculations of exchange interactions between metal centres provide a clear evidence of the role played by the fundamental geometrical factors on the nature and magnitude of the magnetic coupling in these pseudo-polymorphic CPs.
Use of metalloligands [CuL] (H2L = salen type di-Schiff bases) in the formation of heterobimetallic copper(II)-uranyl complexes: photophysical investigations, structural variations, and theoretical calculations.[Pubmed:23786416]
Inorg Chem. 2013 Jul 1;52(13):7508-23.
Five heterobimetallic copper(II)-uranium(VI) complexes [(CuL(1))UO2(NO3)2] (1), [{CuL(1)(CH3CN)}UO2(NO3)2] (2), [{CuL(1)(CH3COCH3)}UO2(NO3)2] (3), [{CuL(2)(CH3CN)}UO2(NO3)2](4), and [{CuL(2)(CH3COCH3)}UO2(NO3)2][{CuL(2)}UO2(NO3)2] (5) have been synthesized by reacting the Cu(II)-derived metalloligands [CuL(1)] and [CuL(2)] (where, H2L(1) = N,N'-bis(alpha-methylsalicylidene)-1,3-propanediamine and H2L(2) = N,N'-bis(salicylidene)-1,3-propanediamine) with UO2(NO3)2.6H2O in 1:1 ratio by varying the reaction temperature and solvents. Absorption and fluorescence quenching experiments (steady-state and time-resolved) indicate the formation of 1:1 ground-state charge transfer copper(II)-uranium(VI) complexes in solution. X-ray single-crystal structure reveals that each complex contains diphenoxido bridged Cu(II)-U(VI) dinuclear core with two chelated nitrato coligands. The complexes are solvated (acetonitrile or acetone) in the axial position of the Cu(II) in different manner or desolvated. The supramolecular interactions that depend upon the co-ordinating metalloligands seem to control the solvation. In complexes 2 and 3 a rare NO3(-)...NO3(-) weak interaction plays an important role in forming supramolecular network whereas an uncommon U horizontal lineO...NO3(-) weak interaction helps to self-assemble heterobinuclear units in complex 5. The significance of the noncovalent interactions in terms of energies and geometries has been analyzed using theoretical calculations.
Systematic study on the structures of salen type lanthanide complexes tuned by lanthanide contraction and corresponding luminescence.[Pubmed:23660709]
Dalton Trans. 2013 Jul 14;42(26):9482-9.
Two types of N,N'-bis(salicylidene)-1,3-propanediamine (H2L) lanthanide complexes, viz. [Ln(NO3)3(H2L)2].0.2CH3OH [Ln = La (1), Ce (2) and Pr (3)] and [Ln(NO3)3(H2L)2]2.CH2Cl2.CH3OH [Ln = Nd (4), Sm (5), Eu (6), Gd (7), Tb (8) and Yb (9)], have been isolated by reactions of H2L with Ln(NO3)3.6H2O. X-ray crystallographic and PXRD analysis reveal that 1-3 are isomorphic possessing a novel one-dimensional (1D) ladder-like double-chain structure. Complexes 4-9 are isostructural exhibiting a discrete dinuclear structure. Luminescent analysis reveals the lanthanide ion and ligand-centered co-luminescence for 5, 6 and 8, which are attributed to the incomplete energy transfer from the triplet state of H2L to the resonance energy level of the corresponding Ln(iii) ion. Further, the characteristic near infrared (NIR) luminescence of Nd(iii) and Yb(iii) ions for complexes 4 and 9 have been revealed.
Electrostatic effects on proton coupled electron transfer in oxomanganese complexes inspired by the oxygen-evolving complex of photosystem II.[Pubmed:23570540]
J Phys Chem B. 2013 May 23;117(20):6217-26.
The influence of electrostatic interactions on the free energy of proton coupled electron transfer in biomimetic oxomanganese complexes inspired by the oxygen-evolving complex (OEC) of photosystem II (PSII) are investigated. The reported study introduces an enhanced multiconformer continuum electrostatics (MCCE) model, parametrized at the density functional theory (DFT) level with a classical valence model for the oxomanganese core. The calculated pKa's and oxidation midpoint potentials (E(m)'s) match experimental values for eight complexes, indicating that purely electrostatic contributions account for most of the observed couplings between deprotonation and oxidation state transitions. We focus on pKa's of terminal water ligands in [Mn(II/III)(H2O)6](2+/3+) (1), [Mn(III)(P)(H2O)2](3-) (2, P = 5,10,15,20-tetrakis(2,6-dichloro-3-sulfonatophenyl)porphyrinato), [Mn2(IV,IV)(mu-O)2(terpy)2(H2O)2](4+) (3, terpy = 2,2':6',2''-terpyridine), and [Mn3(IV,IV,IV)(mu-O)4(phen)4(H2O)2](4+) (4, phen = 1,10-phenanthroline) and the pKa's of mu-oxo bridges and Mn E(m)'s in [Mn2(mu-O)2(bpy)4] (5, bpy = 2,2'-bipyridyl), [Mn2(mu-O)2(salpn)2] (6, salpn = N,N'-bis(salicylidene)-1,3-propanediamine), [Mn2(mu-O)2(3,5-di(Cl)-salpn)2] (7), and [Mn2(mu-O)2(3,5-di(NO2)-salpn)2] (8). The analysis of complexes 6-8 highlights the strong coupling between electron and proton transfers, with any Mn oxidation lowering the pKa of an oxo bridge by 10.5 +/- 0.9 pH units. The model also accounts for changes in the E(m)'s by ligand substituents, such as found in complexes 6-8, due to the electron withdrawing Cl (7) and NO2 (8). The reported study provides the foundation for analysis of electrostatic effects in other oxomanganese complexes and metalloenzymes, where proton coupled electron transfer plays a fundamental role in redox-leveling mechanisms.
Differences in nuclearity, molecular shapes, and coordination modes of azide in the complexes of Cd(II) and Hg(II) with a "metalloligand" [CuL] (H2L = N,N'-bis(salicylidene)-1,3-propanediamine): characterization in solid and in solutions, and theoretical calculations.[Pubmed:23131113]
Inorg Chem. 2012 Nov 19;51(22):12407-18.
Two new heterometallic copper(II)-mercury(II) complexes [(CuL)Hg(N3)2]n (1) and [(CuL)2Hg(N3)2] (2) and one copper(II)-cadmium(II) complex [(CuL)2Cd(N3)2] (3) have been synthesized using "metalloligand" [CuL] (where H2L = N,N'-bis(salicylidene)-1,3-propanediamine) and structurally characterized. Complex 1 is a one-dimensional (1D) helical coordination polymer constructed by the joining of the dinuclear [(CuL)Hg(N3)2] units through a single mu-l,l azido bridge. In the dinuclear unit the Hg(II) is bonded with two phenoxido oxygen atoms of "metalloligand" [CuL] and two nitrogen atoms of azido ligands. Complex 2 is a linear trinuclear entity, in which two terminal "metalloligands" [CuL] are coordinated to central Hg(II) through double phenoxido bridges. The azido ligands link the central mercury atom with the terminal copper atoms via mu-l,3 bridges. In contrast, the trinuclear complex 3 is bent. Here, in addition to two double phenoxido bridges, central Cd(II) is bonded to two mutually cis nitrogen atoms of two terminal azido ligands. The variation in the coordination modes of the azido ligand seems to be responsible for the different molecular shapes of 2 and 3. Interestingly, bond distances between the Hg atoms and the central nitrogen atom of the azido ligands are 2.790(4) and 2.816(5) A in 1 and 2.823(4) A in 2. These bond distances are significantly less than the sum of van der Waals radii of mercury (2.04 A) and nitrogen (1.55 A) and considerably longer than the sum of their covalent radii (2.03 A). However the distances are similar to reported Hg-N bond distances of some Hg(II) complexes. Therefore, we have performed a theoretical density functional theory study to know whether there is any interaction between the central nitrogen atom of the azido ligand and the mercury atoms. We have used the Bader's "atoms-in-molecules", energetic and orbital analyses to conclude that such interaction does not exist. The probable reason for different molecular shapes observed in trinuclear complexes of 2 and 3 also has been studied and explained by theoretical calculations and using the CSD. Electronic spectra, EPR spectra and ESI mass spectra show that all three complexes lose their solid state identity in solution.


