1,2-BenzenediolCAS# 120-80-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 120-80-9 | SDF | Download SDF |
| PubChem ID | 289 | Appearance | White powder |
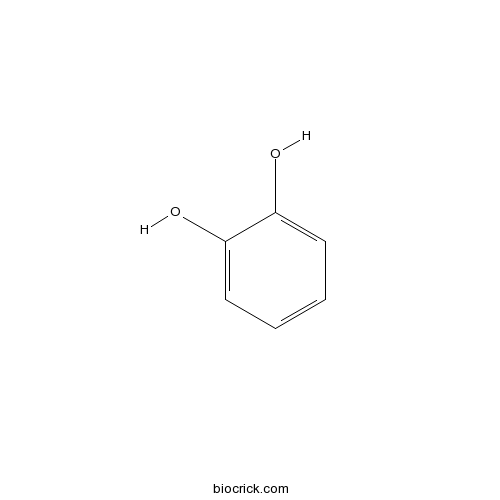
| Formula | C6H6O2 | M.Wt | 110.1 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | 1,2-Benzenediol; 1,2-Dihydroxybenzene; 2-Hydroxyphenol; Pyrocatechin; Pyrocatechol | ||
| Solubility | Soluble in water | ||
| Chemical Name | benzene-1,2-diol | ||
| SMILES | C1=CC=C(C(=C1)O)O | ||
| Standard InChIKey | YCIMNLLNPGFGHC-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1,2-Dihydroxybenzene can induce spontaneous convulsive activity in the anaesthetized mouse and produce myoclonic jerks in the rat. It regulated 5-Hydroxytryptamine (5-HT) levels. |
| Targets | 5-HT Receptor |
| In vivo | A pharmacological study of the spontaneous convulsive activity induced by 1,2-dihydroxybenzene (catechol) in the anaesthetized mouse.[Reference: WebLink]Brit. J. Pharmacol., 1977, 61(3):433-9.
|
| Kinase Assay | Carbonic anhydrase inhibitors: guaiacol and catechol derivatives effectively inhibit certain human carbonic anhydrase isoenzymes (hCA I, II, IX and XII).[Pubmed: 25373500]J Enzyme Inhib Med Chem. 2015 Aug;30(4):586-91.A series of phenolic compounds, including guaiacol, 4-methylguaiacol, 4-propylguaiacol, eugenol, isoeugenol, vanillin, syringaldehyde, 1,2-Benzenediol, 3-methyl catechol, 4-methyl 1,2-Benzenediol and 3-methoxy catechol were investigated for their inhibition of all the catalytically active mammalian isozymes of the Zn(2+)-containing CA (EC 4.2.1.1). All the phenolic compounds effectively inhibited human carbonic anhydrase isoenzymes (hCA I, II, IX and XII), with Kis in the range of 2.20-515.98 μM. The various isozymes showed diverse inhibition profiles. Among the tested phenolic derivatives, compounds 4-methyl 1,2-Benzenediol and 3-methoxy 1,2-Benzenediol showed potent activity as inhibitors of the tumour-associated transmembrane isoforms (hCA IX and XII) in the submicromolar range, with high selectivity. The results obtained from this research may lead to the design of more effective carbonic anhydrase isoenzyme inhibitors (CAIs) based on such phenolic compound scaffolds. |
| Animal Research | An analysis of the myoclonic jerks produced by 1, 2-dihydroxybenzene in the rat.[Reference: WebLink]Electroencephalography & Clinical Neurophysiology, 1973, 35(6):589-601.
|

1,2-Benzenediol Dilution Calculator

1,2-Benzenediol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 9.0827 mL | 45.4133 mL | 90.8265 mL | 181.653 mL | 227.0663 mL |
| 5 mM | 1.8165 mL | 9.0827 mL | 18.1653 mL | 36.3306 mL | 45.4133 mL |
| 10 mM | 0.9083 mL | 4.5413 mL | 9.0827 mL | 18.1653 mL | 22.7066 mL |
| 50 mM | 0.1817 mL | 0.9083 mL | 1.8165 mL | 3.6331 mL | 4.5413 mL |
| 100 mM | 0.0908 mL | 0.4541 mL | 0.9083 mL | 1.8165 mL | 2.2707 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N,N'-Bis(salicylidene)-1,3-propanediamine
Catalog No.:BCC9063
CAS No.:120-70-7
- 2'-Methylacetanilide
Catalog No.:BCC8581
CAS No.:120-66-1
- Isosafrole
Catalog No.:BCC3976
CAS No.:120-58-1
- Benzyl benzoate
Catalog No.:BCN8521
CAS No.:120-51-4
- Ethylparaben
Catalog No.:BCN6094
CAS No.:120-47-8
- Desoxyanisoin
Catalog No.:BCN2264
CAS No.:120-44-5
- 3-Amino-4-methoxybenzanilide
Catalog No.:BCC8613
CAS No.:120-35-4
- Clorofene
Catalog No.:BCC8919
CAS No.:120-32-1
- Tropine benzilate
Catalog No.:BCN1921
CAS No.:3736-36-5
- Veratraldehyde
Catalog No.:BCN6089
CAS No.:120-14-9
- Scoparone
Catalog No.:BCN6088
CAS No.:120-08-1
- Sulfuretin
Catalog No.:BCN4725
CAS No.:120-05-8
- Dichlorphenamide
Catalog No.:BCC3761
CAS No.:120-97-8
- Donepezil HCl
Catalog No.:BCC4569
CAS No.:120011-70-3
- VCH-916
Catalog No.:BCC2031
CAS No.:1200133-34-1
- Edgeworin
Catalog No.:BCN6561
CAS No.:120028-43-5
- Meridinol
Catalog No.:BCN6087
CAS No.:120051-54-9
- Shizukanolide F
Catalog No.:BCN6411
CAS No.:120061-96-3
- CRF (6-33)
Catalog No.:BCC5791
CAS No.:120066-38-8
- Fmoc-D-Arg(Mtr)-OH
Catalog No.:BCC3078
CAS No.:120075-24-3
- Flavanthrin
Catalog No.:BCN3687
CAS No.:120090-80-4
- IPI-145 (INK1197)
Catalog No.:BCC1104
CAS No.:1201438-56-3
- Crotaleschenine
Catalog No.:BCN2077
CAS No.:120154-95-2
- Vinflunine Tartrate
Catalog No.:BCC4602
CAS No.:1201898-17-0
Reaction Kinetics of Catechol (1,2-Benzenediol) and Guaiacol (2-Methoxyphenol) with Ozone.[Pubmed:26053029]
J Phys Chem A. 2015 Jul 2;119(26):6759-65.
The kinetic reactions of 1,2-Benzenediol (catechol) and 2-methoxyphenol (guaiacol) with ozone were studied in a simulation chamber (8 m(3)) under dark conditions. The rate coefficients were measured at 294 +/- 2 K, atmospheric pressure and dry conditions (relative humidity, RH < 1%), except for 1,2-Benzenediol where they were also measured as a function of relative humidity (RH = 1-80%). The concentrations of organic compounds were followed by a PTR-ToF-MS for a continuous monitoring of gas-phase species. The O3 rate coefficients were obtained using both the pseudo-first-order and relative rate methods. The values (in cm(3) molecule(-1) s(-1)) determined for catechol and guaiacol under dry conditions are (13.5 +/- 1.1) x 10(-18) and (0.40 +/- 0.31) x 10(-18), respectively. The rate coefficient of catechol was found to be independent of RH below 20% and above 60%, whereas for RH between 20% and 60% it decreases with increasing RH. The determined rate coefficients have been used to evaluate the atmospheric lifetime of each compound with respect to O3. To our knowledge, this study represents the first determination of the ozone rate coefficient with guaiacol and is also the first kinetic investigation for the influence of the relative humidity on the oxygenated aromatic ozonolysis.
Carbonic anhydrase inhibitors: guaiacol and catechol derivatives effectively inhibit certain human carbonic anhydrase isoenzymes (hCA I, II, IX and XII).[Pubmed:25373500]
J Enzyme Inhib Med Chem. 2015;30(4):586-91.
Carbonic anhydrases (CAs) are widespread metalloenzymes in higher vertebrates including humans. A series of phenolic compounds, including guaiacol, 4-methylguaiacol, 4-propylguaiacol, eugenol, isoeugenol, vanillin, syringaldehyde, catechol, 3-methyl catechol, 4-methyl catechol and 3-methoxy catechol were investigated for their inhibition of all the catalytically active mammalian isozymes of the Zn(2+)-containing CA (EC 4.2.1.1). All the phenolic compounds effectively inhibited human carbonic anhydrase isoenzymes (hCA I, II, IX and XII), with Kis in the range of 2.20-515.98 muM. The various isozymes showed diverse inhibition profiles. Among the tested phenolic derivatives, compounds 4-methyl catechol and 3-methoxy catechol showed potent activity as inhibitors of the tumour-associated transmembrane isoforms (hCA IX and XII) in the submicromolar range, with high selectivity. The results obtained from this research may lead to the design of more effective carbonic anhydrase isoenzyme inhibitors (CAIs) based on such phenolic compound scaffolds.


