EthylparabenCAS# 120-47-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 120-47-8 | SDF | Download SDF |
| PubChem ID | 8434 | Appearance | Powder |
| Formula | C9H10O3 | M.Wt | 166.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | Ethyl parahydroxybenzoate; Ethyl 4-hydroxybenzoate | ||
| Solubility | DMSO : 100 mg/mL (601.79 mM; Need ultrasonic) | ||
| Chemical Name | ethyl 4-hydroxybenzoate | ||
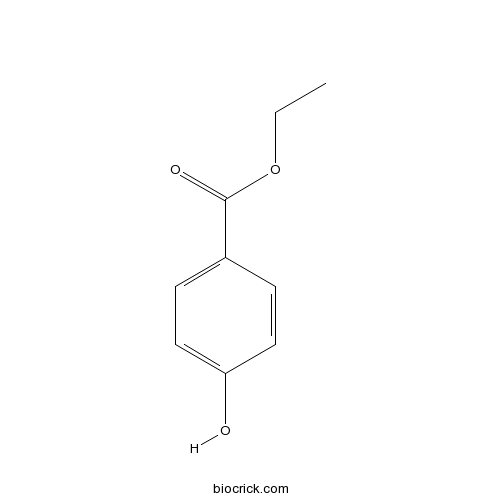
| SMILES | CCOC(=O)C1=CC=C(C=C1)O | ||
| Standard InChIKey | NUVBSKCKDOMJSU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H10O3/c1-2-12-9(11)7-3-5-8(10)6-4-7/h3-6,10H,2H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ethylparaben is the ethyl ester of p-hydroxybenzoic acid, used as an antifungal preservative and food additive. It is a standardized chemical allergen, has a certain reproductive toxicity to F0 male Drosophila.The physiologic effect of ethylparaben is by means of Increased Histamine Release, and Cell-mediated Immunity. |
| Targets | Antifection |
| In vitro | Identification of ethylparaben as the antimicrobial substance produced by Brevibacillus brevis FJAT-0809-GLX.[Pubmed: 25542595]Microbiol Res. 2015 Mar;172:48-56.
Ethylparaben affects lifespan, fecundity, and the expression levels of ERR, EcR and YPR in Drosophila melanogaster.[Pubmed: 25265034]J Insect Physiol. 2014 Dec;71:1-7.Parabens, which mainly include mEthylparaben (MP), Ethylparaben (EP), propylparaben (PP), and butylparaben (BP), are widely used as cosmetic and food preservatives.
Although these chemicals, when used as preservatives, are thought to be safe for humans, many studies have demonstrated that they have estrogenic effects, and can affect the normal development and functions of the reproductive systems in a number of animal species.
|
| Animal Research | [Reproductive toxicity of ethylparaben on male Drosophila melanogaster].[Pubmed: 24868981]Wei Sheng Yan Jiu. 2014 Mar;43(2):265-70.To investigate the reproductive toxicity of different concentration of Ethylparaben (EP) on male Drosophila.
|
| Structure Identification | Indian J Pharm Sci. 2010 Jul;72(4):421-5.A New Validated HPLC Method for the Simultaneous Determination of 2-phenoxyethanol, Methylparaben, Ethylparaben and Propylparaben in a Pharmaceutical Gel.[Pubmed: 21218050]
|

Ethylparaben Dilution Calculator

Ethylparaben Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.0168 mL | 30.0842 mL | 60.1685 mL | 120.3369 mL | 150.4212 mL |
| 5 mM | 1.2034 mL | 6.0168 mL | 12.0337 mL | 24.0674 mL | 30.0842 mL |
| 10 mM | 0.6017 mL | 3.0084 mL | 6.0168 mL | 12.0337 mL | 15.0421 mL |
| 50 mM | 0.1203 mL | 0.6017 mL | 1.2034 mL | 2.4067 mL | 3.0084 mL |
| 100 mM | 0.0602 mL | 0.3008 mL | 0.6017 mL | 1.2034 mL | 1.5042 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ethylparaben is the ethyl ester of p-hydroxybenzoic acid, used as an antifungal preservative. and food additive
- Desoxyanisoin
Catalog No.:BCN2264
CAS No.:120-44-5
- 3-Amino-4-methoxybenzanilide
Catalog No.:BCC8613
CAS No.:120-35-4
- Clorofene
Catalog No.:BCC8919
CAS No.:120-32-1
- Tropine benzilate
Catalog No.:BCN1921
CAS No.:3736-36-5
- Veratraldehyde
Catalog No.:BCN6089
CAS No.:120-14-9
- Scoparone
Catalog No.:BCN6088
CAS No.:120-08-1
- Sulfuretin
Catalog No.:BCN4725
CAS No.:120-05-8
- Unedone
Catalog No.:BCN6759
CAS No.:1199815-09-2
- INT-777
Catalog No.:BCC5390
CAS No.:1199796-29-6
- 1,2-Didehydrotanshinone IIA
Catalog No.:BCN3143
CAS No.:119963-50-7
- Peroxy Orange 1
Catalog No.:BCC6336
CAS No.:1199576-10-7
- 7-O-Methylmorroniside
Catalog No.:BCN7293
CAS No.:119943-46-3
- Benzyl benzoate
Catalog No.:BCN8521
CAS No.:120-51-4
- Isosafrole
Catalog No.:BCC3976
CAS No.:120-58-1
- 2'-Methylacetanilide
Catalog No.:BCC8581
CAS No.:120-66-1
- N,N'-Bis(salicylidene)-1,3-propanediamine
Catalog No.:BCC9063
CAS No.:120-70-7
- 1,2-Benzenediol
Catalog No.:BCN6103
CAS No.:120-80-9
- Dichlorphenamide
Catalog No.:BCC3761
CAS No.:120-97-8
- Donepezil HCl
Catalog No.:BCC4569
CAS No.:120011-70-3
- VCH-916
Catalog No.:BCC2031
CAS No.:1200133-34-1
- Edgeworin
Catalog No.:BCN6561
CAS No.:120028-43-5
- Meridinol
Catalog No.:BCN6087
CAS No.:120051-54-9
- Shizukanolide F
Catalog No.:BCN6411
CAS No.:120061-96-3
- CRF (6-33)
Catalog No.:BCC5791
CAS No.:120066-38-8
Ethylparaben affects lifespan, fecundity, and the expression levels of ERR, EcR and YPR in Drosophila melanogaster.[Pubmed:25265034]
J Insect Physiol. 2014 Dec;71:1-7.
Parabens, which mainly include mEthylparaben (MP), Ethylparaben (EP), propylparaben (PP), and butylparaben (BP), are widely used as cosmetic and food preservatives. Although these chemicals, when used as preservatives, are thought to be safe for humans, many studies have demonstrated that they have estrogenic effects, and can affect the normal development and functions of the reproductive systems in a number of animal species. By treating fruit flies (Drosophila melanogaster) with EP, here we show that lower concentration of EP (0.02%) enhanced fertility while higher concentration of EP (0.10% and 0.20%) shortened the lifespan and reduced the fecundity of fruit flies. When we analyzed the expression levels of the estrogen-related receptor gene (ERR), ecdysone receptor gene (EcR) and Yolk protein receptor gene (YPR) from control and EP-treated fruit flies by using quantitative real-time PCR, we found that the expression levels of all three genes were significantly changed by EP treatment, and that female fruit flies are more sensitive to EP than males. Our data suggests that the estrogenic and the toxic effects of EP to fruit flies may have a molecular basis through the hormonal effect of EP.
A New Validated HPLC Method for the Simultaneous Determination of 2-phenoxyethanol, Methylparaben, Ethylparaben and Propylparaben in a Pharmaceutical Gel.[Pubmed:21218050]
Indian J Pharm Sci. 2010 Jul;72(4):421-5.
A novel reversed-phase HPLC method has been developed and validated for the simultaneous determination of 2-phenoxyethanol, mEthylparaben, Ethylparaben and propylparaben preservatives. The method uses a Lichrosorb C8 (150x4.6 mm, 5 microm) column and isocratic elution. The mobile phase consisted of a mixture of acetonitrile, tetrahydrofuran and water (21:13:66, v/v/v), pumped at a flow rate of 1 ml/min. The UV detection was set at 258 nm. The method was validated with respect to accuracy, precision (repeatability and intermediate precision), specificity, linearity and range. All the parameters examined met the current recommendations for bioanalytical method validation. The developed method was successfully applied to the determination of commercially available pharmaceutical gel products for these preservatives. The procedure describes here is simple, selective and reliable for routine quality control analysis and stability tests.
Identification of ethylparaben as the antimicrobial substance produced by Brevibacillus brevis FJAT-0809-GLX.[Pubmed:25542595]
Microbiol Res. 2015 Mar;172:48-56.
In this study, crude antimicrobial extract from the culture supernatant of Brevibacillus brevis FJAT-0809-GLX was extracted, and its antimicrobial activity was investigated with the agar diffusion method. The results showed that the antimicrobial activity of the culture supernatant of B. brevis FJAT-0809-GLX increased with the extension of the incubation time of B. brevis FJAT-0809-GLX. The antimicrobial spectrum assays showed that this crude antimicrobial extract from culture supernatant of B. brevis FJAT-0809-GLX could inhibit the growth of both bacteria and fungi. A heat stability test was performed, and different temperatures (30 degrees C, 50 degrees C and 70 degrees C) did not affect the antibiotic activity of this crude antimicrobial extract. The crude antimicrobial extract was also tolerable to changes in pH levels. Its antibiotic activity against Escherichia coli was stable at pH 1 to pH 11, with zone sizes ranging from 18.46mm to 22.19mm. Almost all of the crude extracts extracted using different solvents showed variable degrees of inhibition zones against E. coli, with zone sizes ranging from 17.29mm to 19.62mm, except petroleum ether and butanol extracts, which were found to be completely inactive. Purification of the antimicrobial components was carried out using a column chromatographic technique with column chromatography grade silica gel and analyzed by an Agilent 7890A Network GC system. The separated compound was identified as Ethylparaben, with a retention time of 21.980min and a relative amount of 95.50%. The antimicrobial activity of Ethylparaben on different types of bacteria and fungi was investigated, and Ethylparaben was shown to inhibit different types of microbes to different extents. To the best of our knowledge, this is the first report demonstrating that the bacterium B. brevis could produce Ethylparaben.
[Reproductive toxicity of ethylparaben on male Drosophila melanogaster].[Pubmed:24868981]
Wei Sheng Yan Jiu. 2014 Mar;43(2):265-70.
OBJECTIVE: To investigate the reproductive toxicity of different concentration of Ethylparaben (EP) on male Drosophila. METHODS: New eclosion flies within 8h in the basal medium were collected and male Drosophila among them were put into control group and EP treating groups with different concentration (0.03%, 0.07% and 0.10%) by random selection. Female Drosophila was all put into control group. After cultured for 5 days by one pair in one tube and 10 times of repetition for each group, the egg laying amount and maximum egg laying amount of 10d parental generation (F0) and first filial generation (F1), the emergence rate and emergence amount of F1 and second finial generation (F2), the duration time of eggs-arvae, larvae-pupa, and pupa-adult flies, and the total duration time of emergence were counted. RESULTS: The egg laying amount, emergence rate and emergence amount of EP treated parental Drosophila were significantly lower than those of the control group (P < 0.01). The total duration time of emergence of F1 prolongs with the increase of EP concentration. The egg laying amount of F1 and emergence rate of F2 in high EP concentration group had remarkable different compared to those of control group (P < 0.01). And the total duration time of F2 had also shortened significantly compared to that of control group (P < 0.05), while there was no remarkable difference in 0.03% and 0.07% concentration group. CONCLUSION: EP has a certain reproductive toxicity to F0 male Drosophila and EP of high concentration can impact sustainable to offspring which has some facilitation effect on F1 Drosophila.


