DichlorphenamideCAS# 120-97-8 |
- S0859
Catalog No.:BCC1914
CAS No.:1019331-10-2
- Sodium Channel inhibitor 1
Catalog No.:BCC1959
CAS No.:1198117-23-5
- Nav1.7 inhibitor
Catalog No.:BCC4191
CAS No.:1355631-24-1
- Riluzole
Catalog No.:BCC3849
CAS No.:1744-22-5
- Flecainide acetate
Catalog No.:BCC1578
CAS No.:54143-56-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 120-97-8 | SDF | Download SDF |
| PubChem ID | 3038 | Appearance | Powder |
| Formula | C6H6Cl2N2O4S2 | M.Wt | 305.16 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Diclofenamide | ||
| Solubility | DMSO : 100 mg/mL (327.70 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
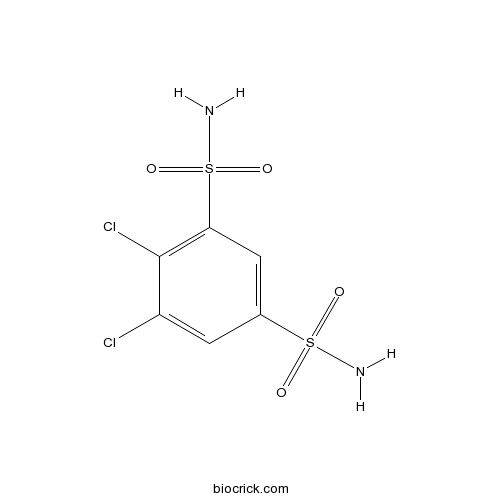
| Chemical Name | 4,5-dichlorobenzene-1,3-disulfonamide | ||
| SMILES | C1=C(C=C(C(=C1S(=O)(=O)N)Cl)Cl)S(=O)(=O)N | ||
| Standard InChIKey | GJQPMPFPNINLKP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dichlorphenamide(Diclofenamide) is a carbonic anhydrase inhibitor that is used in the treatment of glaucoma.
Target: Carbonic Anhydrase
Dichlorphenamide is a sulfonamide and a carbonic anhydrase inhibitor of the meta-Disulfamoylbenzene class. This drug has the same side-effects as acetazolamide, for which it is a useful substitute, except for a lesser tendency to cause dermatitis, renal calculi and metabolic acidosis. It may induce a more pronounced renal loss of potassium [1]. An average daily dose of 33 mg of diclofenamide, a carbonic-anhydrase inhibitor, was added to the anti-epileptic medication already employed in 105 cases of severe epilepsy which had shown insufficient clinical improvement. A favourable action on seizures, often accompanied by an improvement in the EEG tracing, was observed in 83 cases. The effect was of long duration in 47 cases in that it lasted for more than a year. It persisted for one to twelve months in a further 17 cases, while in 19 patients, who had reacted favourably to the treatment, medication had to be suspended because of intolerance [2]. References: | |||||

Dichlorphenamide Dilution Calculator

Dichlorphenamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.277 mL | 16.3848 mL | 32.7697 mL | 65.5394 mL | 81.9242 mL |
| 5 mM | 0.6554 mL | 3.277 mL | 6.5539 mL | 13.1079 mL | 16.3848 mL |
| 10 mM | 0.3277 mL | 1.6385 mL | 3.277 mL | 6.5539 mL | 8.1924 mL |
| 50 mM | 0.0655 mL | 0.3277 mL | 0.6554 mL | 1.3108 mL | 1.6385 mL |
| 100 mM | 0.0328 mL | 0.1638 mL | 0.3277 mL | 0.6554 mL | 0.8192 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Dichlorphenamide is a carbonic anhydrase inhibitor. Carbonic anhydrase is a protein in your body.
- 1,2-Benzenediol
Catalog No.:BCN6103
CAS No.:120-80-9
- N,N'-Bis(salicylidene)-1,3-propanediamine
Catalog No.:BCC9063
CAS No.:120-70-7
- 2'-Methylacetanilide
Catalog No.:BCC8581
CAS No.:120-66-1
- Isosafrole
Catalog No.:BCC3976
CAS No.:120-58-1
- Benzyl benzoate
Catalog No.:BCN8521
CAS No.:120-51-4
- Ethylparaben
Catalog No.:BCN6094
CAS No.:120-47-8
- Desoxyanisoin
Catalog No.:BCN2264
CAS No.:120-44-5
- 3-Amino-4-methoxybenzanilide
Catalog No.:BCC8613
CAS No.:120-35-4
- Clorofene
Catalog No.:BCC8919
CAS No.:120-32-1
- Tropine benzilate
Catalog No.:BCN1921
CAS No.:3736-36-5
- Veratraldehyde
Catalog No.:BCN6089
CAS No.:120-14-9
- Scoparone
Catalog No.:BCN6088
CAS No.:120-08-1
- Donepezil HCl
Catalog No.:BCC4569
CAS No.:120011-70-3
- VCH-916
Catalog No.:BCC2031
CAS No.:1200133-34-1
- Edgeworin
Catalog No.:BCN6561
CAS No.:120028-43-5
- Meridinol
Catalog No.:BCN6087
CAS No.:120051-54-9
- Shizukanolide F
Catalog No.:BCN6411
CAS No.:120061-96-3
- CRF (6-33)
Catalog No.:BCC5791
CAS No.:120066-38-8
- Fmoc-D-Arg(Mtr)-OH
Catalog No.:BCC3078
CAS No.:120075-24-3
- Flavanthrin
Catalog No.:BCN3687
CAS No.:120090-80-4
- IPI-145 (INK1197)
Catalog No.:BCC1104
CAS No.:1201438-56-3
- Crotaleschenine
Catalog No.:BCN2077
CAS No.:120154-95-2
- Vinflunine Tartrate
Catalog No.:BCC4602
CAS No.:1201898-17-0
- MLN9708
Catalog No.:BCC2091
CAS No.:1201902-80-8
Dichlorphenamide: A Review in Primary Periodic Paralyses.[Pubmed:26941026]
Drugs. 2016 Mar;76(4):501-7.
Oral Dichlorphenamide (Keveyis) is a carbonic anhydrase inhibitor that is approved in the USA for the treatment of primary hyperkalaemic and hypokalaemic periodic paralyses and related variants. The efficacy and safety of Dichlorphenamide in patients with primary periodic paralyses have been evaluated in four 9-week, randomized, double-blind, placebo-controlled, phase III trials [two parallel-group trials (HOP and HYP) and two crossover trials]. In two trials in patients with hypokalaemic periodic paralysis, Dichlorphenamide was associated with a significantly (eightfold) lower paralytic attack rate and fewer patients with acute intolerable worsening compared with placebo. In two trials in patients with hyperkalaemic periodic paralysis, the attack rate was lower with Dichlorphenamide than placebo, with this comparison reaching statistical significance in one trial (crossover) but not the other (HYP), although the attack rate was approximately fivefold lower with Dichlorphenamide than placebo in the HYP trial. In 52-week, open-label extensions of the HOP and HYP trials, Dichlorphenamide provided sustained efficacy in patients with hypokalaemic or hyperkalaemic periodic paralysis. Dichlorphenamide was generally well tolerated in all four phase III trials and during the extension trials; the most common adverse events were paraesthesia, cognitive disorders and dysgeusia. As the first agent to be approved in the USA for this indication, Dichlorphenamide is a valuable treatment option for patients with primary hyperkalaemic or hypokalaemic periodic paralysis.
Randomized, placebo-controlled trials of dichlorphenamide in periodic paralysis.[Pubmed:26865514]
Neurology. 2016 Apr 12;86(15):1408-1416.
OBJECTIVE: To determine the short-term and long-term effects of Dichlorphenamide (DCP) on attack frequency and quality of life in hyperkalemic (HYP) and hypokalemic (HOP) periodic paralysis. METHODS: Two multicenter randomized, double-blind, placebo-controlled trials lasted 9 weeks (Class I evidence), followed by a 1-year extension phase in which all participants received DCP. Forty-four HOP and 21 HYP participants participated. The primary outcome variable was the average number of attacks per week over the final 8 weeks of the double-blind phase. RESULTS: The median attack rate was lower in HOP participants on DCP than in participants on placebo (0.3 vs 2.4, p = 0.02). The 9-week mean change in the Physical Component Summary score of the Short Form-36 was also better in HOP participants receiving DCP (treatment effect = 7.29 points, 95% confidence interval 2.26 to 12.32, p = 0.006). The median attack rate was also lower in HYP participants on DCP (0.9 vs 4.8) than in participants on placebo, but the difference in median attack rate was not significant (p = 0.10). There were no significant effects of DCP on muscle strength or muscle mass in either trial. The most common adverse events in both trials were paresthesia (47% DCP vs 14% placebo, both trials combined) and confusion (19% DCP vs 7% placebo, both trials combined). CONCLUSIONS: DCP is effective in reducing the attack frequency, is safe, and improves quality of life in HOP periodic paralysis. CLASSIFICATION OF EVIDENCE: These studies provide Class I evidence that DCP significantly reduces attack frequency in HOP but lacked the precision to support either efficacy or lack of efficacy of DCP in HYP.


