ReynosinCAS# 28254-53-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 28254-53-7 | SDF | Download SDF |
| PubChem ID | 98958 | Appearance | Powder |
| Formula | C15H20O3 | M.Wt | 248.3 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
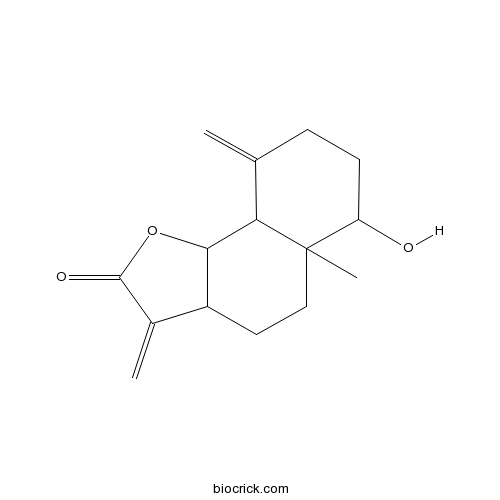
| Chemical Name | 6-hydroxy-5a-methyl-3,9-dimethylidene-3a,4,5,6,7,8,9a,9b-octahydrobenzo[g][1]benzofuran-2-one | ||
| SMILES | CC12CCC3C(C1C(=C)CCC2O)OC(=O)C3=C | ||
| Standard InChIKey | FKBUODICGDOIGB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H20O3/c1-8-4-5-11(16)15(3)7-6-10-9(2)14(17)18-13(10)12(8)15/h10-13,16H,1-2,4-7H2,3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Reynosin has hepatoprotective effects, it can inhibit thioacetamide-induced apoptosis in primary hepatocytes and an in vivo mouse model. 2. Reynosin has protective effect against dopamine-induced neuronal cell death, which may be due to the reciprocal up-regulation of E6-associated protein and down-regulation of α-synuclein protein expression. 3. Reynosin exhibits a dose-dependent inhibition on CINC-1 induction in LPS-stimulated NRK-52E cells, where 50% of inhibitory effect was shown at the concentration of about 1 microM. 4. Reynosin has strong anti-mycobactericidal activity, with a minimal bactericidal concentration (MBC) of 128ug/mL against the H37Rv, 366-2009 and 104-2010 Mtb strains and a minimal inhibitory concentration (MIC) of 64, 64, 128, 128 and 128ug/mL against the H37Rv, 104-2010, 63-2009, 366-2009 and 430-2010 Mtb strains, respectively. 5. Reynosin can inhibit nitric oxide (NO) production in lipopolysaccharide (LPS)-activated murine macrophages. 6. Reynosin shows cytotoxicity against the KB cancer cell line (ATCC CCL17), with IC50 value of 2.7 microg/mL. 7. Reynosin shows moderate anti-inflammatory activity, it exhibits a concentration-related decrease in the levels of IL-1β, IL-6, TNF-α, PGE2, lipoxgenase-5, and COX-2. |
| Targets | Bcl-2/Bax | NO | TNF-α | PGE | LOX | COX | IL Receptor | Antifection |

Reynosin Dilution Calculator

Reynosin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0274 mL | 20.1369 mL | 40.2739 mL | 80.5477 mL | 100.6847 mL |
| 5 mM | 0.8055 mL | 4.0274 mL | 8.0548 mL | 16.1095 mL | 20.1369 mL |
| 10 mM | 0.4027 mL | 2.0137 mL | 4.0274 mL | 8.0548 mL | 10.0685 mL |
| 50 mM | 0.0805 mL | 0.4027 mL | 0.8055 mL | 1.611 mL | 2.0137 mL |
| 100 mM | 0.0403 mL | 0.2014 mL | 0.4027 mL | 0.8055 mL | 1.0068 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- HOOBt
Catalog No.:BCC2817
CAS No.:28230-32-2
- Phlorin
Catalog No.:BCN5177
CAS No.:28217-60-9
- JTE 907
Catalog No.:BCC7380
CAS No.:282089-49-0
- Lauterine
Catalog No.:BCN7062
CAS No.:28200-65-9
- Dihydrodehydrodiconiferyl alcohol
Catalog No.:BCN5176
CAS No.:28199-69-1
- Sinensin
Catalog No.:BCN4797
CAS No.:28189-90-4
- 5-Amino-2-mercaptobenzimidazole
Catalog No.:BCC8730
CAS No.:2818-66-8
- Futoquinol
Catalog No.:BCN6416
CAS No.:28178-92-9
- CHC
Catalog No.:BCC7994
CAS No.:28166-41-8
- Peonidin-3-O-galactoside chloride
Catalog No.:BCN3027
CAS No.:28148-89-2
- 8-Aminoadenine
Catalog No.:BCC6108
CAS No.:28128-33-8
- H-Pro-OtBu
Catalog No.:BCC3020
CAS No.:2812-46-6
- Tyrphostin A1
Catalog No.:BCC5404
CAS No.:2826-26-8
- Baicalein 6-O-glucoside
Catalog No.:BCN3325
CAS No.:28279-72-3
- Beta-Elemonic acid
Catalog No.:BCN2981
CAS No.:28282-25-9
- Daurinoline
Catalog No.:BCN2742
CAS No.:2831-75-6
- 5-Hydroxy-1-tetralone
Catalog No.:BCN8397
CAS No.:28315-93-7
- Valifenalate
Catalog No.:BCC8071
CAS No.:283159-90-0
- Fmoc-ß-HoAsn(Trt)-OH
Catalog No.:BCC3228
CAS No.:283160-20-3
- Rucaparib (free base)
Catalog No.:BCC4012
CAS No.:283173-50-2
- 11beta-Hydroxycedrelone
Catalog No.:BCN5179
CAS No.:283174-18-5
- sn-Glycero-3-phosphocholine
Catalog No.:BCC4168
CAS No.:28319-77-9
- IVHD-valtrate
Catalog No.:BCN7125
CAS No.:28325-56-6
- 6-Ethoxydihydrosanguinarine
Catalog No.:BCN7589
CAS No.:28342-31-6
Reynosin and santamarine: two sesquiterpene lactones from Ambrosia confertiflora with bactericidal activity against clinical strains of Mycobacterium tuberculosis.[Pubmed:27180996]
Pharm Biol. 2016 Nov;54(11):2623-2628.
CONTEXT: Tuberculosis is primarily caused by Mycobacterium tuberculosis (Mtb). Previous studies have shown that the dichloromethanic extract of Ambrosia confertiflora DC (Asteraceae) inhibited Mtb. OBJECTIVE: To isolate the compounds responsible for the mycobactericidal activity against clinical Mtb strains. MATERIALS AND METHODS: The dichloromethanic extract of aerial parts of A. confertiflora was separated using chromatography columns. Mycobactericidal activity of the isolated compounds was evaluated using the Alamar Blue bioassay (128-16 mug/mL, 7 days). Cytotoxicity was tested against normal cell line L929 using the MTT ([3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium]) assay (100-3.125 mug/mL, 48 h). Compound structures were elucidated by (1)H and (13)C uni- and bidimensional NMR. RESULTS: Two sesquiterpene lactones (SQLs) with mycobactericidal activity were identified: santamarine and Reynosin. Reynosin was the most active compound, with a minimal bactericidal concentration (MBC) of 128 mug/mL against the H37Rv, 366-2009 and 104-2010 Mtb strains and a minimal inhibitory concentration (MIC) of 64, 64, 128, 128 and 128 mug/mL against the H37Rv, 104-2010, 63-2009, 366-2009 and 430-2010 Mtb strains, respectively. Santamarine had MBCs of 128 mug/mL against the H3Rv and 104-2010 Mtb strains and MICs of 128 mug/mL against the H37Rv, 366-2009 and 104-2010 Mtb strains. We also isolated 1,10-epoxyparthenolide but only showed mycobacteriostatic activity (MIC 128 mug/mL) against the Mtb strain. Compounds were tested against the L929 cell line and the calculated selectivity index was <1. DISCUSSION AND CONCLUSIONS: This is the first report of the mycobactericidal activity of these compounds against clinical Mtb strains. It is also the first report of the isolation of 1,10-epoxyparthenolide from A. confertiflora. The anti-mycobacterial activity of A. confertiflora was attributed to the SQLs identified.
New cytotoxic sesquiterpene lactones from Warionia saharae.[Pubmed:12802731]
Planta Med. 2003 May;69(5):462-4.
Cytotoxicity-guided fractionation of the methanol soluble part of the dichloromethane extract of the leaves of Warionia saharae led to the isolation of the two new guaianolide-type sesquiterpene lactones, 5 alpha H-3 beta,4 beta-epoxy-14-oxo-guaia-1(10),11(13)-dien-6 alpha,12-olide (1), 5 alpha H-2 beta,4 beta-epoxy-3 alpha-hydroxy-guaia-1(10),11(13)-dien-6 alpha,12-olide (6), and the new eudesmane type sesquiterpene 1 beta,6 alpha-dihydroxycostic acid ( 4). In addition, the known sesquiterpene lactones 5 alpha H-2 beta-hydroxyguaia-3(4),10(14),11(13)-trien-6 alpha,12-olide (2), Reynosin (3), 5 alpha H-1 alpha,10 alpha:3 alpha,4 alpha-diepoxyguaia-11(13)-en-6 alpha,12-olide (5), and dehydroleucodin (7) were isolated together with the known flavone hispidulin. Cytotoxicity testing of the sesquiterpene lactones against the KB cancer cell line (ATCC CCL17) revealed IC50 values of 3.5 (1), 2.6 ( 2), 2.7 ( 3), 4.3 ( 5), 3.6 ( 6), and 1.3 (7) microg/mL. Compound 4 was not active up to 20 microg/mL.
New sesquiterpene lactones from Laurus nobilis leaves as inhibitors of nitric oxide production.[Pubmed:16142632]
Planta Med. 2005 Aug;71(8):706-10.
Two new metabolites 5alphaH,7alphaH-eudesman-4alpha,6alpha,11,12-tetraol (1) and 1beta,15-dihydroxy-5alphaH,7alphaH-eudesma-3,11(13)-dien-12,6alpha-olide ( 2) have been isolated from the methanolic extract of Laurus nobilis L. leaves. Their structures were determined through analysis of their one- and two-dimensional NMR spectral data ((1)H- and (13)C-NMR, DEPT, COSY, HMQC, HMBC and ROESY). The relative stereochemistry is proposed on the basis of combined J-based configuration analysis and ROESY data. In addition, three known sesquiterpene lactones santamarine (3), Reynosin (4) and costunolide (5) along with blumenol C (6) were isolated and identified by spectral means. The isolated compounds 1 - 6 were found to inhibit nitric oxide (NO) production in lipopolysaccharide (LPS)-activated murine macrophages. The most active compound 2 potently inhibited NO (2)(-) release with an IC (50) value of 0.8 microM.
Anti-inflammatory sesquiterpenes from Costus speciosus rhizomes.[Pubmed:26593213]
J Ethnopharmacol. 2015 Dec 24;176:365-74.
ETHNOPHARMACOLOGICAL RELEVANCE: Costus speciosus (Koen ex. Retz.) Sm. (crepe ginger, family Costaceae) is an ornamental plant used in traditional medicine for the treatment of inflammation, rheumatism, bronchitis, fever, headache, asthma, flatulence, constipation, helminthiasis, leprosy, skin diseases, hiccough, anemia, as well as burning sensation on urination. AIM OF THE STUDY: The present study is designed to isolate and identify the active compounds from C. speciosus rhizomes and measure their anti-inflammatory activities. MATERIALS AND METHODS: The n-hexane-CHCl3 soluble fraction of the MeOH extract of C. speciosus rhizomes has been subjected to a repeated column chromatography, including normal silica gel and RP-18 column to give eight compounds. The structures of these compounds were established by UV, IR, 1D ((1)H and (13)C), and 2D ((1)H-(1)H COSY, NOESY, HSQC, and HMBC) NMR experiments and HRESIMS data. In addition, the anti-inflammatory activity of compounds 1-8 was evaluated by measuring the levels IL-6, IL-1beta, TNF-alpha, COX-2, lipoxgenase-5, and PGE2 using enzyme-linked immunosorbent assay. RESULTS: The n-hexane-CHCl3 soluble fraction afforded a new eudesmane acid, specioic acid (8), along with seven known compounds, 22,23-dihydrospinasterone (1), dehydrodihydrocostus lactone (mokko lactone) (2), dehydrocostus lactone (3), stigmasterol (4), arbusculin A (5), santamarine (douglanin) (6), and Reynosin (7). Compounds 1, 4, and 5-7 were isolated for the first time C. speciosus. Compounds 1-4 displayed potent anti-inflammatory activity, while 7 and 8 showed moderate activity. Compounds 1-8 exhibited a concentration-related decrease in the levels of IL-1beta, IL-6, TNF-alpha, PGE2, lipoxgenase-5, and COX-2. Compounds 5 and 6 did not significantly decrease levels of different cytokines, PGE2, lipoxgenase-5, and COX-2 from PHA treatment at 1 microM. However, all tested compounds significantly decreased cytokines, PGE2, lipoxgenase-5, and COX-2 levels at concentration 100 microM. It is noteworthy that compounds 1-4 had the highest activity, where it lowered levels of cytokines, PGE2, lipoxgenase-5, and COX-2 to the extent that was no statistical difference from the control group. Thus, they decreased proinflammatory cytokines (IL-1beta, IL-6, and TNF-alpha) with decreased level of the target enzymes (COX-2 and lipoxgenase-5) and subsequent reduction of its inflammatory product (PGE2). CONCLUSION: Good anti-inflammatory activities exhibited of the isolated compounds from C. speciosus corroborate the usefulness of this plant in the traditional treatment of inflammation and related symptoms.
Hepatoprotective effects of reynosin against thioacetamide-induced apoptosis in primary hepatocytes and mouse liver.[Pubmed:23435943]
Arch Pharm Res. 2013 Apr;36(4):485-94.
The aim of this study was to identify the hepatoprotective effects of Reynosin, sesquiterpenes from the leaves of Laurus nobilis, against thioacetamide (TAA)-induced apoptosis in primary hepatocyte cultures and an in vivo mouse model. Rat hepatocytes were isolated and pretreated with 0.13, 0.64, or 3.22 muM Reynosin and then exposed to 100 mM TAA. Reynosin treatment significantly inhibited TAA-induced apoptosis and hepatocellular DNA damage in primary rat hepatocytes. We observed an increase in levels of antiapoptotic Bcl-2, Bcl-XL mRNA and a decrease in levels of proapoptotic Bax mRNA following Reynosin treatment of hepatocytes. Apoptosis in BALB/c mice was induced with intra-peritoneal injection of 200 mg/kg TAA for 2 weeks every other day. Then Reynosin (5 mg/kg) and TAA were intragastrically given for 3 weeks every other day. Aspartate aminotransferase and alanine aminotransferase levels in the blood of mice were decreased in the Reynosin administration group. Bcl-2 and Bcl-XL mRNA levels were increased, and the Bax mRNA level was decreased in Reynosin-treated mice. Thus, Reynosin inhibited TAA-induced apoptosis in primary hepatocytes and an in vivo mouse model.
Reynosin protects against neuronal toxicity in dopamine-induced SH-SY5Y cells and 6-hydroxydopamine-lesioned rats as models of Parkinson's disease: Reciprocal up-regulation of E6-AP and down-regulation of alpha-synuclein.[Pubmed:23751361]
Brain Res. 2013 Aug 2;1524:54-61.
Aggregation of alpha-synuclein (ASYN) is considered a major determinant of neuronal loss in Parkinson's disease (PD). E6-associated protein (E6-AP), an E3 ubiquitin protein ligase, has been known to promote the degradation of alpha-synuclein. The aim of this study was to assess the effects of the sesquiterpene lactone Reynosin on dopamine (DA)-induced neuronal toxicity and regulation of E6-associated protein and alpha-synuclein proteins in both in vitro and in vivo models of Parkinson's disease. Usi"ng flow cytometry and western blot analysis, we determined that Reynosin significantly protected both against cell death from dopamine-induced toxicity in human neuroblastoma SH-SY5Y cells and against the loss of tyrosine hydroxylase (TH)-positive cells in 6-hydroxydopamine (6-OHDA)-lesioned rats (a rodent Parkinson's disease model system). In addition, Reynosin made up-regulation of E6-associated protein expression and down-regulation of the over-expression of alpha-synuclein protein in both dopamine-treated SH-SY5Y cells and 6-hydroxydopamine-lesioned rats. These results suggest that the protective effect of Reynosin against dopamine-induced neuronal cell death may be due to the reciprocal up-regulation of E6-associated protein and down-regulation of alpha-synuclein protein expression.


