HOOBtCAS# 28230-32-2 |
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- BMS-345541
Catalog No.:BCC1423
CAS No.:547757-23-3
- Bay 65-1942 free base
Catalog No.:BCC1408
CAS No.:600734-02-9
- Bay 65-1942 HCl salt
Catalog No.:BCC1409
CAS No.:600734-06-3
- Bay 65-1942 R form
Catalog No.:BCC1410
CAS No.:758683-21-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 28230-32-2 | SDF | Download SDF |
| PubChem ID | 73026 | Appearance | Powder |
| Formula | C7H5N3O2 | M.Wt | 163.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in water or 1% acetic acid | ||
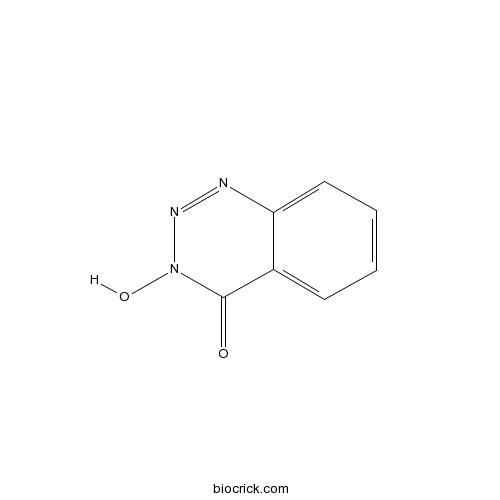
| Chemical Name | 3-hydroxy-1,2,3-benzotriazin-4-one | ||
| SMILES | C1=CC=C2C(=C1)C(=O)N(N=N2)O | ||
| Standard InChIKey | HJBLUNHMOKFZQX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H5N3O2/c11-7-5-3-1-2-4-6(5)8-9-10(7)12/h1-4,12H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

HOOBt Dilution Calculator

HOOBt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.1312 mL | 30.656 mL | 61.3121 mL | 122.6242 mL | 153.2802 mL |
| 5 mM | 1.2262 mL | 6.1312 mL | 12.2624 mL | 24.5248 mL | 30.656 mL |
| 10 mM | 0.6131 mL | 3.0656 mL | 6.1312 mL | 12.2624 mL | 15.328 mL |
| 50 mM | 0.1226 mL | 0.6131 mL | 1.2262 mL | 2.4525 mL | 3.0656 mL |
| 100 mM | 0.0613 mL | 0.3066 mL | 0.6131 mL | 1.2262 mL | 1.5328 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
HOOBt
- Phlorin
Catalog No.:BCN5177
CAS No.:28217-60-9
- JTE 907
Catalog No.:BCC7380
CAS No.:282089-49-0
- Lauterine
Catalog No.:BCN7062
CAS No.:28200-65-9
- Dihydrodehydrodiconiferyl alcohol
Catalog No.:BCN5176
CAS No.:28199-69-1
- Sinensin
Catalog No.:BCN4797
CAS No.:28189-90-4
- 5-Amino-2-mercaptobenzimidazole
Catalog No.:BCC8730
CAS No.:2818-66-8
- Futoquinol
Catalog No.:BCN6416
CAS No.:28178-92-9
- CHC
Catalog No.:BCC7994
CAS No.:28166-41-8
- Peonidin-3-O-galactoside chloride
Catalog No.:BCN3027
CAS No.:28148-89-2
- 8-Aminoadenine
Catalog No.:BCC6108
CAS No.:28128-33-8
- H-Pro-OtBu
Catalog No.:BCC3020
CAS No.:2812-46-6
- Pluviatolide
Catalog No.:BCN3041
CAS No.:28115-68-6
- Reynosin
Catalog No.:BCN5178
CAS No.:28254-53-7
- Tyrphostin A1
Catalog No.:BCC5404
CAS No.:2826-26-8
- Baicalein 6-O-glucoside
Catalog No.:BCN3325
CAS No.:28279-72-3
- Beta-Elemonic acid
Catalog No.:BCN2981
CAS No.:28282-25-9
- Daurinoline
Catalog No.:BCN2742
CAS No.:2831-75-6
- 5-Hydroxy-1-tetralone
Catalog No.:BCN8397
CAS No.:28315-93-7
- Valifenalate
Catalog No.:BCC8071
CAS No.:283159-90-0
- Fmoc-ß-HoAsn(Trt)-OH
Catalog No.:BCC3228
CAS No.:283160-20-3
- Rucaparib (free base)
Catalog No.:BCC4012
CAS No.:283173-50-2
- 11beta-Hydroxycedrelone
Catalog No.:BCN5179
CAS No.:283174-18-5
- sn-Glycero-3-phosphocholine
Catalog No.:BCC4168
CAS No.:28319-77-9
- IVHD-valtrate
Catalog No.:BCN7125
CAS No.:28325-56-6
Efficient preparation of Fmoc-aminoacyl-N-ethylcysteine unit, a key device for the synthesis of peptide thioesters.[Pubmed:21842100]
Org Biomol Chem. 2011 Oct 7;9(19):6807-13.
The synthesis of Fmoc-aminoacyl-N-ethyl-S-triphenylmethylcysteine, an N- to S-acyl migratory device for the preparation of peptide thioesters by Fmoc-SPPS (solid-phase peptide synthesis) is described. Condensation of Fmoc-aminoacyl pentafluorophenyl ester and N-ethyl-S-triphenylmethylcysteine was efficiently performed in the presence of HOOBt (3-hydroxy-3,4-dihydro-4-oxo-1,2,3-benzotriazine) in DMF. A small amount of diastereomer yielded during the reaction was easily separated by HPLC purification and the highly pure devices were obtained for most of the proteinogenic amino acids.
Synthesis of biantennary complex-type nonasaccharyl asn building blocks for solid-phase glycopeptide synthesis.[Pubmed:21612260]
J Org Chem. 2011 Jul 1;76(13):5229-39.
The biantennary complex-type N-glycans bearing LacNAc and LacdiNAc as the nonreducing end motif were synthesized in a protected form suitable to use in the Fmoc solid-phase peptide synthesis studies. Two approaches for the nonasaccharide synthesis were examined by taking advantage of the highly beta-selective glycosylation with GlcNTCA (N-phenyl)trifluoroacetimidate. An earlier approach, which involved the reaction of the trisaccharide donor (Gal-GlcNTCA-Man) and trisaccharide acceptor (Man-GlcNPhth(2)-N(3)), produced a mixture of nonasaccharide isomers. On the other hand, mannosylation of the trisaccharide acceptor (Man-GlcNPhth(2)-N(3)) stereoselectively afforded the known pentasaccharide (Man(3)-GlcNPhth(2)-N(3)), which was reacted with the disaccharyl glycosyl donor (Gal-GlcNTCA or GalNTCA-GlcNTCA) to produce the desired nonasaccharide as a single stereoisomer. Selective dephthaloylation followed by N-acetylation furnished the GlcNAc(2) functionality. The resulting nonasaccharyl azides were condensed with Fmoc-Asp(OPfp)-OBu(t) or Fmoc-Asp(OPfp)-OPac in the presence of Ph(CH(3))(2)P and HOOBt. Finally, the Zn reduction and cleavage of the tert-butyl ester or Zn reduction alone produced the targeted nonasaccharyl Asn building blocks.
Synthesis of a membrane protein with two transmembrane regions.[Pubmed:11991206]
J Pept Sci. 2002 Apr;8(4):172-80.
A membrane protein with two transmembrane domains was synthesized by means of the thioester method. The F1F0 ATP synthase subunit c (Sub.c), which consists of 79 amino acid residues (MW 8257), was chosen as a target. For synthetic purposes, two building blocks, Boc-[Lys34(Boc)]-Sub.c(1-38)-SCH2CH2CO-Ala and Sub.c(39-79), were synthesized via solid-phase methods using Boc chemistry. RP-HPLC purification conditions for the transmembrane peptide were examined. As a result, a combination of a mixture of formic acid, 1-propanol and water with a phenyl column was found to be useful for separating the transmembrane peptide. The purified building blocks were condensed in DMSO in the presence of silver chloride, 3,4-dihydro-3-hydroxy-4-oxo-1,2,3-benzotriazine (HOOBt), N,N-diisopropylethylamine to give the product, Sub.c, after removal of Boc groups (yield 16%). The yield of the condensation reaction could be improved to 23% by raising the reaction temperature to 50 degrees C, and to 26% when a mixture of chloroform and methanol was used as a solvent.
Aza-cycloisodityrosine analogue of RA-VII, an antitumor bicyclic hexapeptide.[Pubmed:24268554]
Bioorg Med Chem Lett. 2013 Dec 15;23(24):6728-31.
An aza-cycloisodityrosine analogue of RA-VII, 3, was designed and synthesized. The key aza-cycloisodityrosine unit was prepared by copper(II)-acetate-mediated intramolecular phenylamine/arylboronic acid coupling of dipeptide followed by connection with the tetrapeptide segment to afford a hexapeptide. Subsequent macrocyclization of the hexapeptide with EDC . HCl and HOOBt under dilute conditions gave 3. Analogue 3 showed significant cytotoxic activity against human promyelocytic leukemia HL-60 cells and human colon carcinoma HCT-116 cells, but its activity was weaker than that of parent peptide RA-VII (1).
Determination of gibberellins in soybean using tertiary amine labeling and capillary electrophoresis coupled with electrochemiluminescence detection.[Pubmed:24184838]
J Chromatogr B Analyt Technol Biomed Life Sci. 2013 Dec 15;941:62-8.
A novel sensitive method based on tertiary amine labeling for the analysis of gibberellins (GAs) by capillary electrophoresis (CE) coupled with electrochemiluminescence (ECL) detection was proposed. GA3 was tagged with 2-(2-aminoethyl)-1-methylpyrrolidine (AEMP) using N, N'-dicyclohexylcarbodiimide (DCC) and 3,4-dihydro-3-hydroxy-4-oxo-1,2,3-benzotriazine (HOOBt) as coupling agents in acetonitrile to produce GA3-AEMP-derivative. The GA3-AEMP-derivative was injected into CE by electrokinetic injection and detected by Ru(bpy)3(2+)-based ECL. The parameters affecting derivatization, detection and separation such as concentration of reactants, detection potential, pH and concentration of separation buffer, were investigated in detail. Under optimum conditions, the linear concentration range for GA3 was from 2.0x10(-7) to 1.28x10(-4)M with a correlation coefficient of 0.9997. The detection limit was 8x10(-8)M (S/N=3). The relative standard deviations of migration time, peak intensity and peak area for nine continuous injections of 2.0x10(-5)M GA3-AEMP-derivative were 1.0%, 2.1% and 4.2%, respectively. The developed approach was successfully applied to the determination of total GAs in the stem, leaf and seed of soybean (Glycine max [L.] Merr.) with recoveries in the range from 89.6% to 99.3%.


