DaurinolineCAS# 2831-75-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2831-75-6 | SDF | Download SDF |
| PubChem ID | 12309092 | Appearance | Powder |
| Formula | C37H42N2O6 | M.Wt | 610.7 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
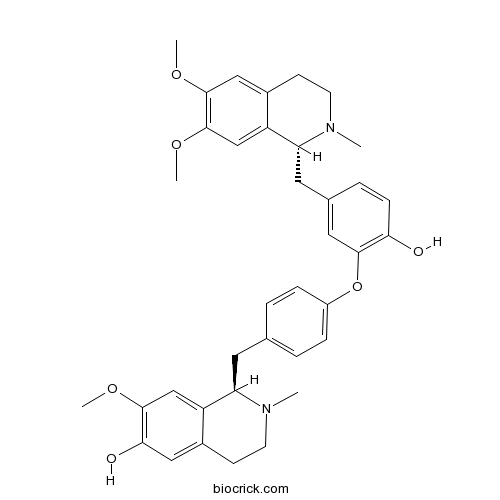
| Chemical Name | (1R)-1-[[4-[5-[[(1R)-6,7-dimethoxy-2-methyl-3,4-dihydro-1H-isoquinolin-1-yl]methyl]-2-hydroxyphenoxy]phenyl]methyl]-7-methoxy-2-methyl-3,4-dihydro-1H-isoquinolin-6-ol | ||
| SMILES | CN1CCC2=CC(=C(C=C2C1CC3=CC=C(C=C3)OC4=C(C=CC(=C4)CC5C6=CC(=C(C=C6CCN5C)OC)OC)O)OC)O | ||
| Standard InChIKey | APIHNXDZCYDPTF-FIRIVFDPSA-N | ||
| Standard InChI | InChI=1S/C37H42N2O6/c1-38-14-12-25-19-33(41)34(42-3)21-28(25)30(38)16-23-6-9-27(10-7-23)45-35-18-24(8-11-32(35)40)17-31-29-22-37(44-5)36(43-4)20-26(29)13-15-39(31)2/h6-11,18-22,30-31,40-41H,12-17H2,1-5H3/t30-,31-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Daurinoline is a non-competitive antagonist, it exerts marked relaxation effect on basilar artery of rabbits through non-competitive antagonism, it would have a protective function on microcirculation of cerebral pia mater, which may be beneficial to relieve cerebral ischemic injury. Daurinoline could significantly reverse noradrenaline induced constriction of pial arterioles and venules, and increase the number of blood vessels which were decreased by noradrenaline. |
| Targets | 5-HT Receptor | Histamine Receptor | Calcium Channel |
| In vivo | Effects of Daurinoline on Microcirculation of Cerebral Pia Mater in Mice[Reference: WebLink]《Medical Journal of Wuhan University》 2011-01To investigate the effect of Daurinoline on cerebral pia mater microcirculation in mice. |
| Kinase Assay | Relaxant Effect of Daurinoline on Vascular Smooth Muscle of Isolated Rabbit Basilar Artery[Reference: WebLink]《Herald of Medicine》 2014-06To investigate the effect of Daurinoline on basilar artery vascular smooth muscle. |

Daurinoline Dilution Calculator

Daurinoline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6375 mL | 8.1873 mL | 16.3747 mL | 32.7493 mL | 40.9366 mL |
| 5 mM | 0.3275 mL | 1.6375 mL | 3.2749 mL | 6.5499 mL | 8.1873 mL |
| 10 mM | 0.1637 mL | 0.8187 mL | 1.6375 mL | 3.2749 mL | 4.0937 mL |
| 50 mM | 0.0327 mL | 0.1637 mL | 0.3275 mL | 0.655 mL | 0.8187 mL |
| 100 mM | 0.0164 mL | 0.0819 mL | 0.1637 mL | 0.3275 mL | 0.4094 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Beta-Elemonic acid
Catalog No.:BCN2981
CAS No.:28282-25-9
- Baicalein 6-O-glucoside
Catalog No.:BCN3325
CAS No.:28279-72-3
- Tyrphostin A1
Catalog No.:BCC5404
CAS No.:2826-26-8
- Reynosin
Catalog No.:BCN5178
CAS No.:28254-53-7
- HOOBt
Catalog No.:BCC2817
CAS No.:28230-32-2
- Phlorin
Catalog No.:BCN5177
CAS No.:28217-60-9
- JTE 907
Catalog No.:BCC7380
CAS No.:282089-49-0
- Lauterine
Catalog No.:BCN7062
CAS No.:28200-65-9
- Dihydrodehydrodiconiferyl alcohol
Catalog No.:BCN5176
CAS No.:28199-69-1
- Sinensin
Catalog No.:BCN4797
CAS No.:28189-90-4
- 5-Amino-2-mercaptobenzimidazole
Catalog No.:BCC8730
CAS No.:2818-66-8
- Futoquinol
Catalog No.:BCN6416
CAS No.:28178-92-9
- 5-Hydroxy-1-tetralone
Catalog No.:BCN8397
CAS No.:28315-93-7
- Valifenalate
Catalog No.:BCC8071
CAS No.:283159-90-0
- Fmoc-ß-HoAsn(Trt)-OH
Catalog No.:BCC3228
CAS No.:283160-20-3
- Rucaparib (free base)
Catalog No.:BCC4012
CAS No.:283173-50-2
- 11beta-Hydroxycedrelone
Catalog No.:BCN5179
CAS No.:283174-18-5
- sn-Glycero-3-phosphocholine
Catalog No.:BCC4168
CAS No.:28319-77-9
- IVHD-valtrate
Catalog No.:BCN7125
CAS No.:28325-56-6
- 6-Ethoxydihydrosanguinarine
Catalog No.:BCN7589
CAS No.:28342-31-6
- Oxychelerythrine
Catalog No.:BCN4864
CAS No.:28342-33-8
- Canolol
Catalog No.:BCC8371
CAS No.:28343-22-8
- Mahanine
Catalog No.:BCN3176
CAS No.:28360-49-8
- Chrysin 6-C-glucoside
Catalog No.:BCN3324
CAS No.:28368-57-2
Daurinoline suppressed the migration and invasion of chemo-resistant human non-small cell lung cancer cells by reversing EMT and Notch-1 and sensitized the cells to Taxol.[Pubmed:30641414]
Environ Toxicol Pharmacol. 2019 Feb;66:109-115.
Non-small cell lung cancer (NSCLC) is one of the most common malignancies, and Taxol is a cornerstone in the treatment. However, taxol-resistance eventually limits the clinical effects and applications. Daurinoline could restore the sensitivity of resistant MCF-7/adr and KBv200 cells. Whereas, the effect of Daurinoline on the chemo-resistant NSCLC cells and the mechanism has not been elucidated. In this study, Daurinoline was firstly demonstrated that inhibited the proliferation, migration, invasion and EMT phenotype of chemo-resistant NSCLC cells. And these effects were associated with EMT and Notch-1 reversal. Moreover, Daurinoline could significantly enhance the anti-tumor effect of Taxol rather than epirubicin, adriamycin and cisplatin. And the reverse fold (RF) value of Daurinoline was greater than terfenadine reported before. There are little cytotoxic effects of Daurinoline and its derivatives reported by L.W. Fu, et al. (2001). Therefore, Daurinoline may be a potential anti-tumor agent or chemosensitizer for chemo-resistant NSCLC patients.
The interaction of telomeric DNA and C-myc22 G-quadruplex with 11 natural alkaloids.[Pubmed:22480315]
Nucleic Acid Ther. 2012 Apr;22(2):127-36.
Telomeric DNA and C-myc22 are DNA G-quadruplex (G4)-forming sequences associated with tumorigenesis. Ligands that can facilitate the formation and increase the stabilization of G4 can halt tumor cell proliferation and have been regarded as potential anti-cancer drugs. In the present study, we have investigated the interaction of 11 natural alkaloids with G4 formed by telomeric DNA and C-myc22 sequences. Our results indicated that sanguinarine (San), palmatine (Pal), and berberine (Beb) of the first series (S1) can induce the formation of G4 as well as increase the stabilization ability. Daurisoline (S2-1), O-methyldauricine (S2-2), O-diacetyldaurisoline (S2-3), Daurinoline (S2-4), dauricinoline (S2-5), N,N'-dimethyldauricine iodide (S2-6), and N,N'-dimethyldaurisoline iodide (S2-7) of the second series (S2) showed similar stabilization ability. We found that unsaturated ring C, N(+) positively charged centers, and conjugated aromatic rings are key factors to increase the stabilization ability of S1, and we gave some advice on structure modification to S2 through structure-activity study. Besides, we found San and Pal to be cell cycle blocker in G(1). San was speculated to bind to G4 through intercalation or end stacking.
Predictable and linear scale-up of four phenolic alkaloids separation from the roots of Menispermum dauricum using high-performance counter-current chromatography.[Pubmed:20576475]
J Chromatogr B Analyt Technol Biomed Life Sci. 2010 Jul 15;878(22):1929-33.
This paper describes how distribution ratios were used for prediction of peak elution in analytical high-performance counter-current chromatography (HPCCC) to explore the method for separation and purification of bioactive compounds from the roots of Menispermum dauricum. Then important parameters related to HPCCC separations including solvent systems, sample concentration, sample loading volume and flow rate were optimized on an analytical Mini-DE HPCCC and finally linearly scaled up to a preparative Midi-DE HPCCC with nearly the same resolutions and separation time. Four phenolic alkaloids were for the first time obtained by HPCCC separation with a two-phase solvent system composed of petroleum ether-ethyl acetate-ethanol-water (1:2:1:2, v/v). This process produced 131.3 mg daurisolin, 197.1 mg dauricine, 32.4 mg Daurinoline and 14.7 mg dauricicoline with the purity of 97.6%, 96.4%, 97.2% and 98.3%, respectively from 500 mg crude extract of the roots of M. dauricum in a one-step separation. The purities of compounds were determined by high-performance liquid chromatography (HPLC). Their structures were identified by electrospray ionization mass spectrometer (ESI-MS) and nuclear magnetic resonance (NMR).
[Isolation and identification of alkaloids form Menispermum dauricum growing in Xianning].[Pubmed:12569837]
Zhong Yao Cai. 1998 Sep;21(9):456-8.
The alkaloids of rhizoma of Menispermum dauricum DC growing in Xianning have been subjected to isolation and identification. The results showed that its two major constituents, which are only next of dauricine in content, are dauricinoline and Daurinoline, instead of the commonly found daurisoline in the same plant materials from North China.


