FutoquinolCAS# 28178-92-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 28178-92-9 | SDF | Download SDF |
| PubChem ID | 5281817 | Appearance | Oil |
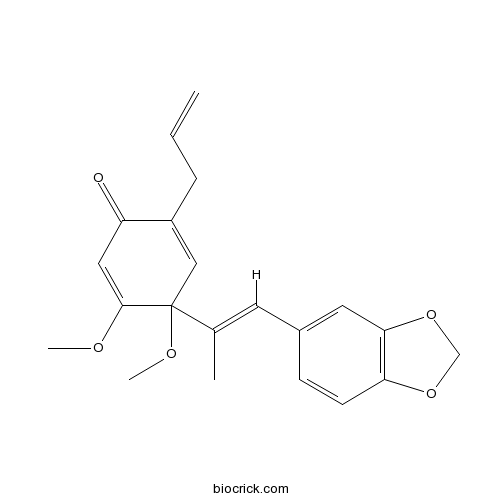
| Formula | C21H22O5 | M.Wt | 354.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-[(E)-1-(1,3-benzodioxol-5-yl)prop-1-en-2-yl]-4,5-dimethoxy-2-prop-2-enylcyclohexa-2,5-dien-1-one | ||
| SMILES | CC(=CC1=CC2=C(C=C1)OCO2)C3(C=C(C(=O)C=C3OC)CC=C)OC | ||
| Standard InChIKey | AOZTYYBGNNXAOI-NTEUORMPSA-N | ||
| Standard InChI | InChI=1S/C21H22O5/c1-5-6-16-12-21(24-4,20(23-3)11-17(16)22)14(2)9-15-7-8-18-19(10-15)26-13-25-18/h5,7-12H,1,6,13H2,2-4H3/b14-9+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Futoquinol can potently inhibit NO production with an IC(50) value of 16.8microM in microglia cells, suggests that it has anti-neuroinflammatory activities. 2. Futoquinol shows potent inhibition of PMA-induced ROS production in human polymorphonuclear neutrophils with IC(50) values 13.1 +/- 5.3 microM. 3. Futoquinol is an anti-platelet activating factor principle from the stem part of Piper futokadsura (Piperaceae), the Chinese drug haifengteng. |
| Targets | NO | ROS | PAFR |

Futoquinol Dilution Calculator

Futoquinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8217 mL | 14.1084 mL | 28.2167 mL | 56.4334 mL | 70.5418 mL |
| 5 mM | 0.5643 mL | 2.8217 mL | 5.6433 mL | 11.2867 mL | 14.1084 mL |
| 10 mM | 0.2822 mL | 1.4108 mL | 2.8217 mL | 5.6433 mL | 7.0542 mL |
| 50 mM | 0.0564 mL | 0.2822 mL | 0.5643 mL | 1.1287 mL | 1.4108 mL |
| 100 mM | 0.0282 mL | 0.1411 mL | 0.2822 mL | 0.5643 mL | 0.7054 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- CHC
Catalog No.:BCC7994
CAS No.:28166-41-8
- Peonidin-3-O-galactoside chloride
Catalog No.:BCN3027
CAS No.:28148-89-2
- 8-Aminoadenine
Catalog No.:BCC6108
CAS No.:28128-33-8
- H-Pro-OtBu
Catalog No.:BCC3020
CAS No.:2812-46-6
- Pluviatolide
Catalog No.:BCN3041
CAS No.:28115-68-6
- Adamantane
Catalog No.:BCN8481
CAS No.:281-23-2
- Chaetocin
Catalog No.:BCC2429
CAS No.:28097-03-2
- (+)-Ulopterol
Catalog No.:BCN1228
CAS No.:28095-18-3
- A 286982
Catalog No.:BCC3946
CAS No.:280749-17-9
- SB 216763
Catalog No.:BCC3650
CAS No.:280744-09-4
- VUF 5574
Catalog No.:BCC7030
CAS No.:280570-45-8
- Rediocide A
Catalog No.:BCN5175
CAS No.:280565-85-7
- 5-Amino-2-mercaptobenzimidazole
Catalog No.:BCC8730
CAS No.:2818-66-8
- Sinensin
Catalog No.:BCN4797
CAS No.:28189-90-4
- Dihydrodehydrodiconiferyl alcohol
Catalog No.:BCN5176
CAS No.:28199-69-1
- Lauterine
Catalog No.:BCN7062
CAS No.:28200-65-9
- JTE 907
Catalog No.:BCC7380
CAS No.:282089-49-0
- Phlorin
Catalog No.:BCN5177
CAS No.:28217-60-9
- HOOBt
Catalog No.:BCC2817
CAS No.:28230-32-2
- Reynosin
Catalog No.:BCN5178
CAS No.:28254-53-7
- Tyrphostin A1
Catalog No.:BCC5404
CAS No.:2826-26-8
- Baicalein 6-O-glucoside
Catalog No.:BCN3325
CAS No.:28279-72-3
- Beta-Elemonic acid
Catalog No.:BCN2981
CAS No.:28282-25-9
- Daurinoline
Catalog No.:BCN2742
CAS No.:2831-75-6
[Anti-platelet activating factor constituents, 2,5-diaryltetrahydrofuran type lignans, from Piper futokadsura Sied. et Zucc].[Pubmed:8216803]
Zhongguo Zhong Yao Za Zhi. 1993 May;18(5):292-4, 318.
Besides the known components kadsurenone, Futoquinol and futoxide, three anti-platelet activating factor principles have been found from the stem part of Piper futokadsura (Piperaceae), the Chinese drug haifengteng. They were separated and identified as galgravin, galbelgin and veraguensin on the basis of HPLC and spectral analysis. The natural existence of these three anti-PAF constituents in haifengteng is reported for the first time.
Anti-inflammatory neolignans from Piper kadsura.[Pubmed:16724856]
J Nat Prod. 2006 May;69(5):842-4.
Two new neolignans, piperkadsin A (1) and piperkadsin B (2), as well as 11 known neolignans, three known alkaloids, the highly oxygenated compound (+)-crotepoxide, and stigmasterol were isolated from the stems of Piper kadsura. The anti-inflammatory activities of these compounds were evaluated. Compounds 1, 2, Futoquinol (3), piperlactam S (4), and N-p-coumaroyl tyramine (5) showed potent inhibition of PMA-induced ROS production in human polymorphonuclear neutrophils with IC(50) values 4.3 +/-1.0, 12.2 +/- 3.2, 13.1 +/- 5.3, 7.0 +/- 1.9, and 8.4 +/- 1.3 microM, respectively.
Neolignans from Piper kadsura and their anti-neuroinflammatory activity.[Pubmed:19900811]
Bioorg Med Chem Lett. 2010 Jan 1;20(1):409-12.
Bioassay-guided column chromatographic separation of the methanolic extract of dried aerial parts of Piper kadsura (Piperaceae) led to the isolation of a new neolignan, piperkadsin C (1), together with eight known neolignans (2-9). The structures of the isolated compounds were elucidated by combined spectroscopic methods. The anti-neuroinflammatory activities of these compounds were evaluated by assessing nitric oxide (NO) production in LPS-activated BV-2 cells, a microglia cell line. Piperkadsin C (1) and Futoquinol (2) potently inhibited NO production with an IC(50) value of 14.6 and 16.8microM in microglia cells, respectively. Compounds 3, 4, 5, 8, and 9 also exhibited moderate inhibition of NO production in BV-2 cells.


