PhlorinCAS# 28217-60-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 28217-60-9 | SDF | Download SDF |
| PubChem ID | 476785 | Appearance | Powder |
| Formula | C12H16O8 | M.Wt | 288.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | Phloroglucinol 1-O-β-D-glucoside | ||
| Solubility | Soluble in methanol and water | ||
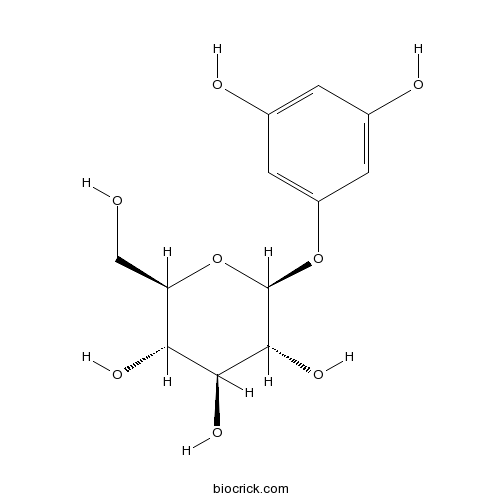
| Chemical Name | (2S,3R,4S,5S,6R)-2-(3,5-dihydroxyphenoxy)-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | C1=C(C=C(C=C1O)OC2C(C(C(C(O2)CO)O)O)O)O | ||
| Standard InChIKey | WXTPOHDTGNYFSB-RMPHRYRLSA-N | ||
| Standard InChI | InChI=1S/C12H16O8/c13-4-8-9(16)10(17)11(18)12(20-8)19-7-2-5(14)1-6(15)3-7/h1-3,8-18H,4H2/t8-,9-,10+,11-,12-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Inorg Chem. 2015 Apr 6;54(7):3501-12.Electrochemical and spectroelectrochemical studies of diphosphorylated metalloporphyrins. Generation of a phlorin anion product.[Pubmed: 25789714]
|

Phlorin Dilution Calculator

Phlorin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4686 mL | 17.343 mL | 34.6861 mL | 69.3722 mL | 86.7152 mL |
| 5 mM | 0.6937 mL | 3.4686 mL | 6.9372 mL | 13.8744 mL | 17.343 mL |
| 10 mM | 0.3469 mL | 1.7343 mL | 3.4686 mL | 6.9372 mL | 8.6715 mL |
| 50 mM | 0.0694 mL | 0.3469 mL | 0.6937 mL | 1.3874 mL | 1.7343 mL |
| 100 mM | 0.0347 mL | 0.1734 mL | 0.3469 mL | 0.6937 mL | 0.8672 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- JTE 907
Catalog No.:BCC7380
CAS No.:282089-49-0
- Lauterine
Catalog No.:BCN7062
CAS No.:28200-65-9
- Dihydrodehydrodiconiferyl alcohol
Catalog No.:BCN5176
CAS No.:28199-69-1
- Sinensin
Catalog No.:BCN4797
CAS No.:28189-90-4
- 5-Amino-2-mercaptobenzimidazole
Catalog No.:BCC8730
CAS No.:2818-66-8
- Futoquinol
Catalog No.:BCN6416
CAS No.:28178-92-9
- CHC
Catalog No.:BCC7994
CAS No.:28166-41-8
- Peonidin-3-O-galactoside chloride
Catalog No.:BCN3027
CAS No.:28148-89-2
- 8-Aminoadenine
Catalog No.:BCC6108
CAS No.:28128-33-8
- H-Pro-OtBu
Catalog No.:BCC3020
CAS No.:2812-46-6
- Pluviatolide
Catalog No.:BCN3041
CAS No.:28115-68-6
- Adamantane
Catalog No.:BCN8481
CAS No.:281-23-2
- HOOBt
Catalog No.:BCC2817
CAS No.:28230-32-2
- Reynosin
Catalog No.:BCN5178
CAS No.:28254-53-7
- Tyrphostin A1
Catalog No.:BCC5404
CAS No.:2826-26-8
- Baicalein 6-O-glucoside
Catalog No.:BCN3325
CAS No.:28279-72-3
- Beta-Elemonic acid
Catalog No.:BCN2981
CAS No.:28282-25-9
- Daurinoline
Catalog No.:BCN2742
CAS No.:2831-75-6
- 5-Hydroxy-1-tetralone
Catalog No.:BCN8397
CAS No.:28315-93-7
- Valifenalate
Catalog No.:BCC8071
CAS No.:283159-90-0
- Fmoc-ß-HoAsn(Trt)-OH
Catalog No.:BCC3228
CAS No.:283160-20-3
- Rucaparib (free base)
Catalog No.:BCC4012
CAS No.:283173-50-2
- 11beta-Hydroxycedrelone
Catalog No.:BCN5179
CAS No.:283174-18-5
- sn-Glycero-3-phosphocholine
Catalog No.:BCC4168
CAS No.:28319-77-9
Electrochemical and spectroelectrochemical studies of diphosphorylated metalloporphyrins. Generation of a phlorin anion product.[Pubmed:25789714]
Inorg Chem. 2015 Apr 6;54(7):3501-12.
Two series of diphosphoryl-substituted porphyrins were synthesized and characterized by electrochemistry and spectroelectrochemistry in nonaqueous media containing 0.1 M tetra-n-butylammonium perchlorate (TBAP). The investigated compounds are 5,15-bis(diethoxyphosphoryl)-10,20-diphenylporphyrins (Ph)2(P(O)(OEt)2)2PorM and 5,15-bis(diethoxyphosphoryl)-10,20-di(para-carbomethoxyphenyl)porphyrins (PhCOOMe)2(P(O)(OEt)2)2PorM where M = 2H, Co(II), Ni(II), Cu(II), Zn(II), Cd(II), or Pd(II). The free-base and five metalated porphyrins with nonredox active centers undergo two ring-centered oxidations and two ring-centered reductions, the latter of which is followed by a chemical reaction of the porphyrin dianion to give an anionic Phlorin product. The Phlorin anion is electroactive and can be reoxidized by two electrons to give back the starting porphyrin, or it can be reversibly reduced by one electron at more negative potentials to give a Phlorin dianion. The chemical conversion of the porphyrin dianion to a Phlorin anion proceeds at a rate that varies with the nature of the central metal ion and the solvent. This rate is slowest in the basic solvent pyridine as compared to CH2Cl2 and PhCN, giving further evidence for the involvement of protons in the chemical reaction leading to Phlorin formation. Calculations of the electronic structure were performed on the Ni(II) porphyrin dianion, and the most favorable atoms for electrophilic attack were determined to be the two phosphorylated carbon atoms. Phlorin formation was not observed after the two-electron reduction of the cobalt porphyrins due to the different oxidation state assignment of the doubly reduced species, a Co(I) pi anion radical in one case and an M(II) dianion for all of the other derivatives. Each redox reaction was monitored by thin-layer UV-visible spectroelectrochemistry, and an overall mechanism for each electron transfer is proposed on the basis of these data.


