AbirateronePotent CYP17 inhibitor CAS# 154229-19-3 |
- PF-4981517
Catalog No.:BCC2270
CAS No.:1390637-82-7
- VT-464
Catalog No.:BCC5398
CAS No.:1610537-15-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 154229-19-3 | SDF | Download SDF |
| PubChem ID | 132971 | Appearance | Powder |
| Formula | C24H31NO | M.Wt | 349.52 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMF : 8.75 mg/mL (25.04 mM; Need ultrasonic and warming) Ethanol : 5.4 mg/mL (15.45 mM; Need ultrasonic) DMSO : 5 mg/mL (14.31 mM; Need ultrasonic) | ||
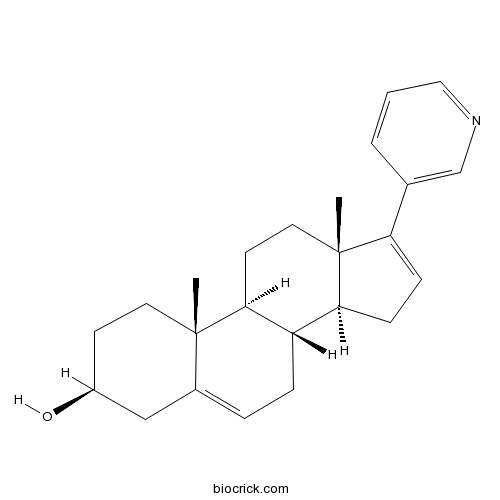
| Chemical Name | (3S,8R,9S,10R,13S,14S)-10,13-dimethyl-17-pyridin-3-yl-2,3,4,7,8,9,11,12,14,15-decahydro-1H-cyclopenta[a]phenanthren-3-ol | ||
| SMILES | CC12CCC(CC1=CCC3C2CCC4(C3CC=C4C5=CN=CC=C5)C)O | ||
| Standard InChIKey | GZOSMCIZMLWJML-VJLLXTKPSA-N | ||
| Standard InChI | InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Abiraterone is a potent inhibitor of CYP17 with IC50 of 2 nM. | |||||
| Targets | CYP17 | |||||
| IC50 | 2 nM | |||||
| Kinase experiment [1]: | |
| C17,20-lyase activity assay | Microsomes were diluted to a final protein concentration of 50 g/ml in the reaction mixture which contained 0.25 M sucrose, 20 mM Tris–HCl (pH 7.4), 10 mM G6P and 1.2 IU/ml G6PDH. After equilibration at 37 ℃ for 10 min, the reaction was initiated by addition of NADP to obtain a final concentration of 0.6 mM. Prior to the distribution of 600 μl of the reaction mixture in each tube, test compounds were evaporated to dryness under a stream of nitrogen and then were incubated at 37 ℃ for 10 minutes. After incubation with Abiraterone, 500 μl of the reaction mixture was transferred to tubes containing 1M of the enzyme substrate, 17OHP. After a further 10 min incubation, tubes were placed on ice and the reaction was stopped by addition of 0.1 ml NaOH 1N. Tubes were deep-frozen and stored at -20℃ until assayed for Δ4A levels. A Δ4A RIA was developed and automated on a microplate format in our laboratory using a specific antibody against Δ4A. The separation of free and bound antigen was achieved with a dextran-coated charcoal suspension. After centrifugation, aliquots of the clear supernatant were counted in duplicates in a 1450 MicrobetaPlus liquid scintillation counter. The Δ4A concentrations of unknown samples were determined from the standard curve. The detection limit was 0.5 ng/ml and the within and between assay coefficients of variation were 10.7 and 17.6%, respectively at an assay value of 13 ng/ml. The rate of enzymatic reaction was expressed as pmol of Δ4A formed per 10 min and per mg of protein. The value of maximum activity without inhibitor (control) was set at 100%. The IC50 values were calculated using non-linear analysis from the plot of enzyme activity (%) against log of inhibitor concentration. |
| Cell experiment [2]: | |
| Cell lines | LNCaP and VCaP cells |
| Preparation method | Limited solubility. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
| Reacting condition | 24 h-96 h |
| Applications | Abiraterone is an inhibitor of CYP17A1 for the treatment of docetaxel-treated castration-resistant prostate cancer. Abiraterone inhibits in vitro proliferation and AR-regulated gene expression of AR-positive prostate cancer cells. |
| Animal experiment [1]: | |
| Animal models | Adult male Wistar rats weighing 220–240 g |
| Dosage form | Administered by oral route at 10 ml/kg once daily for 3 days. |
| Preparation method | Distilled water with a few drops of Tween 80 |
| Application | After 3 days of oral treatment at 50 mg/kg per day, abiraterone acetate markedly inhibits VP (-14%) and SV weights (-37%) without affecting adrenal weight (-7%). It also significantly inhibited T secretion (-48%) and in turn increased LH concentration (192%). |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: 1. Duc I, Bonnet P, Duranti V et al. In vitro and in vivo models for the evaluation of potent inhibitors of male rat 17alpha-hydroxylase/C17,20-lyase. J Steroid Biochem Mol Biol. 2003 Apr;84(5):537-42. 2. Richards J, Lim AC, Hay CW et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res. 2012 May 1;72(9):2176-82. doi: 10.1158/0008-5472.CAN-11-3980. | |

Abiraterone Dilution Calculator

Abiraterone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8611 mL | 14.3053 mL | 28.6107 mL | 57.2213 mL | 71.5267 mL |
| 5 mM | 0.5722 mL | 2.8611 mL | 5.7221 mL | 11.4443 mL | 14.3053 mL |
| 10 mM | 0.2861 mL | 1.4305 mL | 2.8611 mL | 5.7221 mL | 7.1527 mL |
| 50 mM | 0.0572 mL | 0.2861 mL | 0.5722 mL | 1.1444 mL | 1.4305 mL |
| 100 mM | 0.0286 mL | 0.1431 mL | 0.2861 mL | 0.5722 mL | 0.7153 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Abiraterone (17-(3-pyridyl)androsta-5,16-dien-3β-ol) is a potent small-molecule inhibitor of CYP17 complex (17 alpha-monooxygenase) which is a member of the cytochrome P450 family consisting of 17 alpha-hydroxylase and C17,20-lyase and catalyzing the 17 alpha-hydroxylation of intermediates of steroid biosynthesis involved in testerone sysnthesis. Abiraterone inhibits CYP17 complex by binding to the haem iron of CYP17A1, which forms a 60o angle above the haem plane and packs against the central I helix with 3β-OH interacting with aspargine 202 in the F helix. Abiraterone is currently being investigated to treat late stage prostate cancer, in which it consistently suppresses testosterone levels, leads to significant reduction in PSA level, and prolong life in patients with castration-resistant prostate cancer (CRPC).
Reference
Natasha M. DeVore and Emily E. Scott. Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001. Nature 2012; 482: 116-120
Farshid Dayyani, Gary E. Gallick, Christopher J. Logothetis, Paul G. Corn. Novel therapies for metastatic castrate-resistant prostate cancer. JNCI J Natl Cancer Inst (2011); 103(22): 1665-1675
- Abiraterone Acotate
Catalog No.:BCN2184
CAS No.:154229-18-2
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- N-[2-Isopropylthiazol-4-ylmethyl(methyl)carbamoyl]-L-valine
Catalog No.:BCC9067
CAS No.:154212-61-0
- YM 90K hydrochloride
Catalog No.:BCC7455
CAS No.:154164-30-4
- DMAB-anabaseine dihydrochloride
Catalog No.:BCC7301
CAS No.:154149-38-9
- PD 144418 oxalate
Catalog No.:BCC7429
CAS No.:154130-99-1
- 4-Hydroxy-2-(3-methoxypropyl)-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8707
CAS No.:154127-42-1
- 2-(3-Methoxypropyl)-4-oxo-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8480
CAS No.:154127-41-0
- ((2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)methyl)triphenylphosphonium bromide
Catalog No.:BCC8373
CAS No.:154057-58-6
- 3-(Bromomethyl)-2-cyclopropyl-4-(4'-fluorophenyl)quinoline
Catalog No.:BCC8591
CAS No.:154057-56-4
- Isonormangostin
Catalog No.:BCN1687
CAS No.:15404-80-5
- Fuscaxanthone C
Catalog No.:BCN3885
CAS No.:15404-76-9
- Ampalex
Catalog No.:BCC1359
CAS No.:154235-83-3
- CB-5083
Catalog No.:BCC6528
CAS No.:1542705-92-9
- Echistatin, α1 isoform
Catalog No.:BCC5988
CAS No.:154303-05-6
- Capecitabine
Catalog No.:BCN2168
CAS No.:154361-50-9
- Methyl 3-O-feruloylquinate
Catalog No.:BCN3403
CAS No.:154418-15-2
- 5,5'-Dimethoxylariciresinol 4-O-glucoside
Catalog No.:BCN1556
CAS No.:154418-16-3
- Zinc protoporphyrin IX
Catalog No.:BCC6775
CAS No.:15442-64-5
- Lysicamine
Catalog No.:BCN6523
CAS No.:15444-20-9
- Pramanicin
Catalog No.:BCN1853
CAS No.:154445-05-3
- Fmoc-Arg(Pbf)-OH
Catalog No.:BCC3040
CAS No.:154445-77-9
- NU 7026
Catalog No.:BCC3933
CAS No.:154447-35-5
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
Analytical challenges in quantifying abiraterone with LC-MS/MS in human plasma.[Pubmed:28370076]
Biomed Chromatogr. 2017 Nov;31(11).
A method was developed and validated to quantify Abiraterone in human plasma. During assay development, several analytical challenges were encountered: limited stability in patient samples, adsorption to glass, coelution with metabolites and carry-over issues. Limited stability (2 h) was found for Abiraterone in fresh plasma as well as whole blood at ambient temperature. When kept at 2-8 degrees C, Abiraterone in plasma was stable for 24 h and in whole blood for 8 h. Adsorption of Abiraterone to glass materials was addressed by using polypropylene throughout the method. Carry-over was reduced to acceptable limits by incorporating a third mobile phase into the gradient. The chromatographic separation of Abiraterone with its multiple metabolites was addressed by using a longer analytical column and adjusting the gradient. Abiraterone was extracted by protein precipitation, separated on a C18 column with gradient elution and analyzed with tandem quadrupole mass spectrometry in positive ion mode. A stable deuterated isotope was used as the internal standard. The assay ranges from 1 to 500 ng/mL. Within- and-between-day precisions and accuracies were below 13.4% and within 95-102%. This bioanalytical method was successfully validated and applied to determine plasma concentrations of Abiraterone in clinical studies and in regular patient care for patients with metastatic castration-resistant prostate cancer.
Potential drug-drug interactions with abiraterone in metastatic castration-resistant prostate cancer patients: a prevalence study in France.[Pubmed:28361167]
Cancer Chemother Pharmacol. 2017 May;79(5):1051-1055.
PURPOSE: Abiraterone acetate combined with prednisone improves survival in metastatic castration-resistant prostate cancer (mCRPC) patients. This oral anticancer agent may result in drug-drug interactions (DDI). We aimed to evaluate the prevalence of DDI with Abiraterone and the possible determinants for the occurrence of these DDI. METHODS: We performed a single centre retrospective review from electronic medical records of mCRPC patients treated with Abiraterone from 2011 to 2015. Potential DDI with Abiraterone were identified using Micromedex and were categorized by a 4-point scale severity. RESULTS: Seventy-two out of ninety-five mCRPC pts (median age: 77 years [68-82]) had comorbidities. The median number of drugs used per patient was 7 [5-9]. 66 potential DDI with Abiraterone were detected in 49 patients (52%): 39 and 61% were classified as major and moderate DDI, respectively. In the univariate analysis, pain (p < 0.0001), hypo-albuminemia (p = 0.032), and higher ECOG performance status (PS) (p = 0.013) were significantly associated with a higher risk of DDI with Abiraterone. Pain (p < 0.0001) and PS (p = 0.018) remained significant in the multivariate analysis. CONCLUSIONS: Polypharmacy is an issue among mCRPC patients. In our study, half of the patients have potential DDI with Abiraterone. Patients with pain and poor PS are at higher risk of DDI with Abiraterone. A medication review by a pharmacist is of crucial importance to prevent DDI with Abiraterone.
Abiraterone Acetate for Metastatic Prostate Cancer in Patients With Suboptimal Biochemical Response to Hormone Induction.[Pubmed:28358937]
JAMA Oncol. 2017 Nov 9;3(11):e170231.
Importance: Men with metastatic prostate cancer who have a poor response to initial androgen-deprivation therapy (ADT), as reflected by a prostate-specific antigen (PSA) level higher than 4.0 ng/mL after 7 months of ADT, have a poor prognosis, based on historical controls. Objective: To determine the efficacy of Abiraterone acetate with prednisone in these high-risk patients with a suboptimal response to hormonal induction. Design, Setting, and Participants: A phase 2 single-arm study was conducted through the National Clinical Trials Network-Southwest Oncology Group. Eligible patients had metastatic prostate cancer and a PSA level higher than 4.0 ng/mL between 6 and 12 months after starting ADT. The PSA level could be rising or falling at the time of enrollment, but had to be higher than 4.0 ng/mL. No previous chemotherapy or secondary hormonal therapies were allowed, except in patients receiving a standard, first-generation antiandrogen agent with a falling PSA level at the time of enrollment; this therapy was continued in this cohort. Abiraterone acetate, 1000 mg, once daily with prednisone, 5 mg, twice daily was administered to all participants. A total of 41 men were enrolled between the trial's activation on August 9, 2011, and closure on August 1, 2013. Data analysis was conducted from March 21 to November 29, 2016. Interventions: Abiraterone acetate, 1000 mg, once daily by mouth with prednisone, 5 mg, by mouth twice daily. Main Outcomes and Measures: The primary end point was a PSA level of 0.2 ng/mL or lower within 12 months of starting Abiraterone acetate plus prednisone. A partial response (PR) was a secondary end point, defined as a PSA level reduction to lower than 4.0 ng/mL but higher than 0.2 ng/mL. Results: Of the 41 men enrolled, 1 did not receive any protocol treatment and was excluded from analysis. The median (range) age of the 40 participants was 66 (39-85) years. Five (13%) patients achieved a PSA level of 0.2 ng/mL or lower (95% CI, 4%-27%). Thirteen (33%) additional patients achieved a partial response, with a reduction in the PSA level to lower than 4.0 ng/mL but higher than 0.2 ng/mL. Sixteen (40%) patients had no PSA response and 6 (15%) were not assessable and assumed to be nonresponders. The median progression-free survival was 17.5 months (95% CI, 8.6-25.0 months) and the median overall survival was 25.8 months (95% CI, 15.7-25.8 months). There was 1 incident each of grade 4 adverse events of alanine aminotransferase level elevation and rectal hemorrhage. Eleven patients reported grade 3 adverse events. Conclusions and Relevance: This study did not reach its prescribed level of 6 PSA responses of 0.2 ng/mL or lower, although 5 responses were observed. The overall survival and progression-free survival rates observed in this trial are encouraging compared with historical controls. The therapy was generally well tolerated, without any clear signal of any unexpected adverse effects.


