OxychelerythrineCAS# 28342-33-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 28342-33-8 | SDF | Download SDF |
| PubChem ID | 147279 | Appearance | Powder |
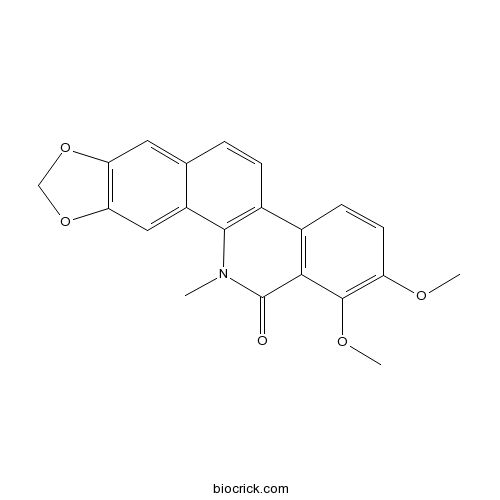
| Formula | C21H17NO5 | M.Wt | 363.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,2-dimethoxy-12-methyl-[1,3]benzodioxolo[5,6-c]phenanthridin-13-one | ||
| SMILES | CN1C2=C(C=CC3=CC4=C(C=C32)OCO4)C5=C(C1=O)C(=C(C=C5)OC)OC | ||
| Standard InChIKey | IHTXRYTWDARUKX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H17NO5/c1-22-19-13(5-4-11-8-16-17(9-14(11)19)27-10-26-16)12-6-7-15(24-2)20(25-3)18(12)21(22)23/h4-9H,10H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Oxychelerythrine shows antifeeding activities against Tribolium castaneum adults, with the EC50 of 192.32 ppm. 2. Oxychelerythrine shows high modulatory activity enhancing the susceptibility of the S. aureus ATCC 6538 to all the tested antibiotics from two to four-fold. |
| Targets | Antifection |

Oxychelerythrine Dilution Calculator

Oxychelerythrine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7518 mL | 13.7589 mL | 27.5179 mL | 55.0358 mL | 68.7947 mL |
| 5 mM | 0.5504 mL | 2.7518 mL | 5.5036 mL | 11.0072 mL | 13.7589 mL |
| 10 mM | 0.2752 mL | 1.3759 mL | 2.7518 mL | 5.5036 mL | 6.8795 mL |
| 50 mM | 0.055 mL | 0.2752 mL | 0.5504 mL | 1.1007 mL | 1.3759 mL |
| 100 mM | 0.0275 mL | 0.1376 mL | 0.2752 mL | 0.5504 mL | 0.6879 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6-Ethoxydihydrosanguinarine
Catalog No.:BCN7589
CAS No.:28342-31-6
- IVHD-valtrate
Catalog No.:BCN7125
CAS No.:28325-56-6
- sn-Glycero-3-phosphocholine
Catalog No.:BCC4168
CAS No.:28319-77-9
- 11beta-Hydroxycedrelone
Catalog No.:BCN5179
CAS No.:283174-18-5
- Rucaparib (free base)
Catalog No.:BCC4012
CAS No.:283173-50-2
- Fmoc-ß-HoAsn(Trt)-OH
Catalog No.:BCC3228
CAS No.:283160-20-3
- Valifenalate
Catalog No.:BCC8071
CAS No.:283159-90-0
- 5-Hydroxy-1-tetralone
Catalog No.:BCN8397
CAS No.:28315-93-7
- Daurinoline
Catalog No.:BCN2742
CAS No.:2831-75-6
- Beta-Elemonic acid
Catalog No.:BCN2981
CAS No.:28282-25-9
- Baicalein 6-O-glucoside
Catalog No.:BCN3325
CAS No.:28279-72-3
- Tyrphostin A1
Catalog No.:BCC5404
CAS No.:2826-26-8
- Canolol
Catalog No.:BCC8371
CAS No.:28343-22-8
- Mahanine
Catalog No.:BCN3176
CAS No.:28360-49-8
- Chrysin 6-C-glucoside
Catalog No.:BCN3324
CAS No.:28368-57-2
- Aloin B
Catalog No.:BCN2576
CAS No.:28371-16-6
- 7-Neohesperidosides
Catalog No.:BCN8200
CAS No.:28383-41-7
- Bumetanide
Catalog No.:BCC1119
CAS No.:28395-03-1
- 1(10)-Aristolen-2-one
Catalog No.:BCN7634
CAS No.:28398-06-3
- FR 236924
Catalog No.:BCC7564
CAS No.:28399-31-7
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- DR 2313
Catalog No.:BCC2451
CAS No.:284028-90-6
- NPS-2143
Catalog No.:BCC4409
CAS No.:284035-33-2
- Ac9-25
Catalog No.:BCC5997
CAS No.:284040-76-2
Synthesis of oxychelerythrine using lithiated toluamide-benzonitrile cycloaddition.[Pubmed:15635245]
Chem Pharm Bull (Tokyo). 2005 Jan;53(1):118-20.
Oxychelerythrine, benzo[c]phenanthridine alkaloid, was synthesized from easily available starting toluamide 5 and benzonitrile 6 using toluamide-benzonitrile cycloaddition reaction in 6 steps.
Enhancing activity of antibiotics against Staphylococcus aureus: Zanthoxylum capense constituents and derivatives.[Pubmed:25925969]
Phytomedicine. 2015 Apr 15;22(4):469-76.
Six compounds (1-6), isolated from the methanol extract of the roots of the African medicinal plant Zanthoxylum capense Thunb. (Rutaceae), and seven ester derivatives (7-13) were evaluated for their antibacterial activities and modulatory effects on the MIC of antibiotics (erythromycin, oxacillin, and tetracycline) and ethidium bromide (EtBr) against a Staphylococcus aureus reference strain (ATCC 6538). Using the same model, compounds 1-13 were also assessed for their potential as efflux pump inhibitors by a fluorometric assay that measures the accumulation of the broad range efflux pump substrate EtBr. Compounds 8 and 11 were further evaluated for their antibacterial, modulatory and EtBr accumulation effects against four additional S. aureus strains, which included two clinical methicillin-resistant S. aureus (MRSA) strains. Compounds (1-13) have not shown antibacterial activity at the concentration ranges tested. When evaluated against S. aureus ATCC 6538, Oxychelerythrine (1) a benzophenanthridine alkaloid, showed the highest modulatory activity enhancing the susceptibility of this strain to all the tested antibiotics from two to four-fold. Ailanthoidiol diacetate (8) and ailanthoidiol di-2-ethylbutanoate (11) were also good modulators when combined with EtBr, increasing the bacteria susceptibility by four and two-fold, respectively. In the EtBr accumulation assay, using ATCC 6538 strain, the phenylpropanoid (+)-ailanthoidiol (6) and most of its ester derivatives (8-11) exhibited higher activity than the positive control verapamil. The highest effects were found for compounds 8 and 11 that also increased the accumulation of EtBr, using S. aureus ATCC 25923 as model. Furthermore, both compounds (8, 11) were able to enhance the ciprofloxacin activity against the MRSA clinical strains tested, causing a reduction of the antibiotic MIC values from two to four-fold. The EtBr accumulation assay revealed that this modulation activity was not due to an inhibition of efflux pumps mechanism. These results suggested that Z. capense constituents may be valuable as leads for restoring antibiotic activity against MRSA strains.


