CITCOSelective CAR agonist CAS# 338404-52-7 |
- Nemorubicin
Catalog No.:BCC4151
CAS No.:108852-90-0
- Batimastat sodium salt
Catalog No.:BCC2075
CAS No.:130464-84-5
- Plumbagin
Catalog No.:BCN2586
CAS No.:481-42-5
- Fluorouracil (Adrucil)
Catalog No.:BCC2135
CAS No.:51-21-8
- Apigenin
Catalog No.:BCN5658
CAS No.:520-36-5
- LY2334737
Catalog No.:BCC4060
CAS No.:892128-60-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 338404-52-7 | SDF | Download SDF |
| PubChem ID | 9600409 | Appearance | Powder |
| Formula | C19H12Cl3N3OS | M.Wt | 436.74 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO and to 5 mM in ethanol | ||
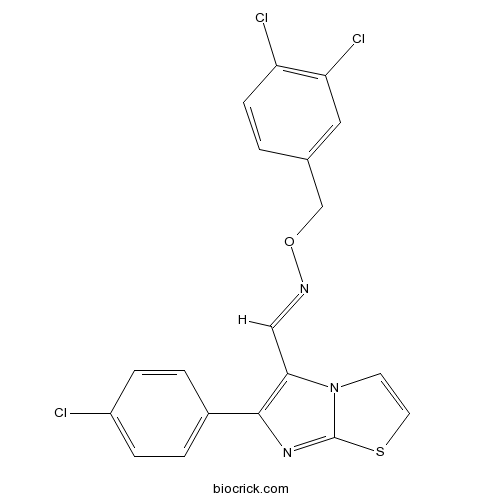
| Chemical Name | (E)-1-[6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazol-5-yl]-N-[(3,4-dichlorophenyl)methoxy]methanimine | ||
| SMILES | C1=CC(=CC=C1C2=C(N3C=CSC3=N2)C=NOCC4=CC(=C(C=C4)Cl)Cl)Cl | ||
| Standard InChIKey | ZQWBOKJVVYNKTL-AUEPDCJTSA-N | ||
| Standard InChI | InChI=1S/C19H12Cl3N3OS/c20-14-4-2-13(3-5-14)18-17(25-7-8-27-19(25)24-18)10-23-26-11-12-1-6-15(21)16(22)9-12/h1-10H,11H2/b23-10+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Constitutive androstane receptor (CAR) agonist (EC50 = 49 nM). Displays > 100-fold selectivity over PXR receptors and no activity at LXR, ERα, ERβ, PPAR, RAR, FXR, VDR and THR. Induces CAR nuclear translocation and expression of CYP2B6 in hepatocytes in vitro. |

CITCO Dilution Calculator

CITCO Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2897 mL | 11.4485 mL | 22.8969 mL | 45.7938 mL | 57.2423 mL |
| 5 mM | 0.4579 mL | 2.2897 mL | 4.5794 mL | 9.1588 mL | 11.4485 mL |
| 10 mM | 0.229 mL | 1.1448 mL | 2.2897 mL | 4.5794 mL | 5.7242 mL |
| 50 mM | 0.0458 mL | 0.229 mL | 0.4579 mL | 0.9159 mL | 1.1448 mL |
| 100 mM | 0.0229 mL | 0.1145 mL | 0.229 mL | 0.4579 mL | 0.5724 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Di-O-methylbergenin
Catalog No.:BCN5263
CAS No.:33815-57-5
- Methasterone
Catalog No.:BCC9027
CAS No.:3381-88-2
- H-D-Abu-OH
Catalog No.:BCC3202
CAS No.:338-69-2
- Deodarin
Catalog No.:BCN6874
CAS No.:33788-39-5
- Hederacholchiside E
Catalog No.:BCC8329
CAS No.:33783-82-3
- Androstanolone heptanoate
Catalog No.:BCC8827
CAS No.:33776-88-4
- Abieta-8,11,13-triene-7,15,18-triol
Catalog No.:BCN5262
CAS No.:337527-10-3
- Cannabivarin
Catalog No.:BCN7587
CAS No.:33745-21-0
- 4,4'-Di-O-methylellagic acid
Catalog No.:BCN3709
CAS No.:3374-77-4
- Voacamine
Catalog No.:BCN8433
CAS No.:3371-85-5
- (-)-Gallocatechin
Catalog No.:BCN5927
CAS No.:3371-27-5
- Pachypodol
Catalog No.:BCN5261
CAS No.:33708-72-4
- LY 456236 hydrochloride
Catalog No.:BCC7347
CAS No.:338736-46-2
- Demethyl tetrandrine
Catalog No.:BCN2624
CAS No.:33889-68-8
- Silychristin
Catalog No.:BCN2389
CAS No.:33889-69-9
- Pabulenol
Catalog No.:BCC8347
CAS No.:33889-70-2
- Mdivi 1
Catalog No.:BCC2402
CAS No.:338967-87-6
- Vandetanib trifluoroacetate
Catalog No.:BCC2029
CAS No.:338992-53-3
- 2,3,4'-Trihydroxy-3',5'-dimethoxypropiophenone
Catalog No.:BCN1455
CAS No.:33900-74-2
- Boc-Ala-OSu
Catalog No.:BCC3048
CAS No.:3392-05-0
- Boc-Gly-OSu
Catalog No.:BCC3397
CAS No.:3392-07-2
- Boc-Ile-Osu
Catalog No.:BCC2604
CAS No.:3392-08-3
- UBP1112
Catalog No.:BCC7033
CAS No.:339526-74-8
- Anamorelin Fumarate
Catalog No.:BCC1363
CAS No.:339539-92-3
Constitutive androstane receptor agonist CITCO inhibits growth and expansion of brain tumour stem cells.[Pubmed:21224854]
Br J Cancer. 2011 Feb 1;104(3):448-59.
BACKGROUND: Brain tumours present unique challenges to conventional therapies and pose major health problems around the world. Brain tumour stem cells (BTSCs) represent a small fraction of tumour cells that maintain growth, drug resistance and recurrence properties. Constitutive androstane receptor (CAR) is a nuclear receptor transcription factor that regulates drug metabolism and homoeostasis. In this study, we examined the effect of CAR agonist, 6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehydeO-(3,4-dichlorobenzyl )oxime (CITCO) on BTSCs. METHODS: The expression of CAR in BTSCs was detected by quantitative RT-PCR and western blot. The antiproliferative effect of CITCO on BTSCs was determined by WST-1 and (3)H thymidine uptake assays. The effect of CITCO on CD133 expression, cell cycle progression and apoptosis in BTSCs was analysed by immunostaining and flow cytometry. The in vivo effect of CITCO was studied using subcutaneous (s.c.) BTSC xenograft in nude mice. RESULTS: We show for the first time that BTSCs express altered levels of nuclear receptors compared with glioma cells. The expression of CAR mRNA and protein was low in BTSCs and that increased following treatment with CITCO in culture. CITCO induced a dose-dependent decrease in growth and expansion of CD133(+) BTSCs as gliospheres in culture. Cell cycle arrest and apoptosis in BTSCs were induced by CITCO, but not in normal astrocytes. Growth of s.c BTSC xenograft in nude mice was also inhibited by CITCO. CONCLUSION: These findings indicate that CITCO inhibits the growth and expansion of BTSCs, suggesting the use of CAR agonists for the treatment of brain tumour.
Constitutive androstane receptor upregulates Abcb1 and Abcg2 at the blood-brain barrier after CITCO activation.[Pubmed:23340159]
Brain Res. 2013 Mar 21;1501:68-80.
ATP-driven efflux transporters are considered to be the major hurdle in the treatment of central nervous system (CNS) diseases. Abcb1 (P-glycoprotein) and Abcg2 (breast cancer resistance protein/brain multidrug resistance protein) belong to the best known ABC-transporters. These ABC-transporters limit the permeability of the blood-brain barrier and protect the brain against toxic compounds in the blood but on the other hand they also reduce the efficacy of CNS pharmacotherapy. Even after 40 years of extensive research, the regulatory mechanisms of these efflux transporters are still not completely understood. To unravel the efflux transporter regulation, we analyzed the effect of the nuclear receptor CAR (constitutive androstane receptor) on the expression of Abcb1 and Abcg2 in primary cultures of porcine brain capillary endothelial cells (PBCEC). CAR is a xenobiotic-activated transcription factor, which is, like the other important nuclear receptor pregnane X receptor (PXR), highly expressed in barrier tissue and known to be a positive regulator of ABC-transporters. We demonstrate that activation of porcine CAR by the human CAR (hCAR) ligand CITCO (6-(4-chlorophenyl)-imidazo[2,1-b]thiazole-5-carbaldehyde) leads to an up-regulation of both transporters, whereas the mouse-specific CAR ligand TCPOBOP (1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene) had no effect on transporter expression. The stimulation of PBCEC with CITCO caused a significant up-regulation of both efflux-transporters on RNA-level, protein level and transport level. Furthermore the additional application of a CAR inhibitor significantly decreased the transporter expression to control niveau. In conclusion our data prove CAR activation only by the human ligand CITCO leading to an increased ABC-transporter expression and transport activity.
A single amino acid controls the functional switch of human constitutive androstane receptor (CAR) 1 to the xenobiotic-sensitive splicing variant CAR3.[Pubmed:19820207]
J Pharmacol Exp Ther. 2010 Jan;332(1):106-15.
The constitutive androstane receptor (CAR) is constitutively activated in immortalized cell lines independent of xenobiotic stimuli. This feature of CAR has limited its use as a sensor for xenobiotic-induced expression of drug-metabolizing enzymes. Recent reports, however, reveal that a splicing variant of human CAR (hCAR3), which contains an insertion of five amino acids (APYLT), exhibits low basal but xenobiotic-inducible activities in cell-based reporter assays. Nonetheless, the underlying mechanisms of this functional shift are not well understood. We have now generated chimeric constructs containing various residues of the five amino acids of hCAR3 and examined their response to typical hCAR activators. Our results showed that the retention of alanine (hCAR1+A) alone is sufficient to confer the constitutively activated hCAR1 to the xenobiotic-sensitive hCAR3. It is noteworthy that hCAR1+A was significantly activated by a series of known hCAR activators, and displayed activation superior to that of hCAR3. Moreover, intracellular localization assays revealed that hCAR1+A exhibits nuclear accumulation upon 6-(4-chlorophenyl) imidazo[2,1-b][1,3]thiazole-5-carbaldehyde-O-(3,4-dichlorobenzyl) oxime (CITCO) treatment in COS1 cells, which differs from the spontaneous nuclear distribution of hCAR1 and the nontranslocatable hCAR3. Mammalian two-hybrid and glutathione S-transferase pull-down assays further demonstrated that hCAR1+A interacts with the coactivator SRC-1 and GRIP-1 at low level before activation, while at significantly enhanced level in the presence of CITCO. Thus, the alanine residue in the insertion of hCAR3 seems in charge of the xenobiotic response of hCAR3 through direct and indirect mechanisms. Activation of hCAR1+A may represent a sensitive avenue for the identification of hCAR activators.
Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes.[Pubmed:12611900]
J Biol Chem. 2003 May 9;278(19):17277-83.
The orphan nuclear constitutive androstane receptor (CAR) is proposed to play a central role in the response to xenochemical stress. Identification of CAR target genes in humans has been limited by the lack of a selective CAR agonist. We report the identification of 6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime (CITCO) as a novel human CAR agonist with the following characteristics: (a) potent activity in an in vitro fluorescence-based CAR activation assay; (b) selectivity for CAR over other nuclear receptors, including the xenobiotic pregnane X receptor (PXR); (c) the ability to induce human CAR nuclear translocation; and (d) the ability to induce the prototypical CAR target gene CYP2B6 in primary human hepatocytes. Using primary cultures of human hepatocytes, the effects of CITCO on gene expression were compared with those of the PXR ligand rifampicin. The relative expression of a number of genes encoding proteins involved in various aspects of steroid and xenobiotic metabolism was analyzed. Notably, CAR and PXR activators differentially regulated the expression of several genes, demonstrating that these two nuclear receptors subserve overlapping but distinct biological functions in human hepatocytes.


