UBP1112Group III mGlu antagonist CAS# 339526-74-8 |
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 339526-74-8 | SDF | Download SDF |
| PubChem ID | 5022489 | Appearance | Powder |
| Formula | C10H14NO5P | M.Wt | 259.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. NaOH | ||
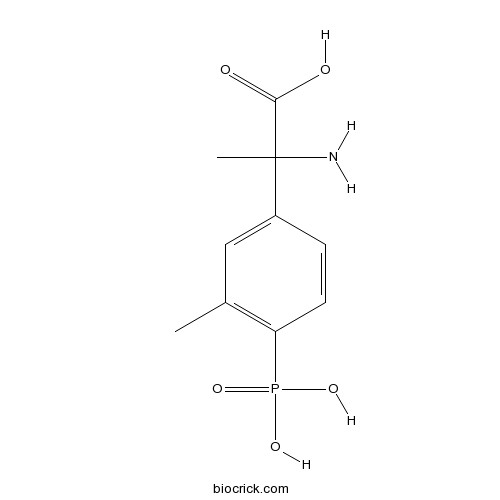
| Chemical Name | 2-amino-2-(3-methyl-4-phosphonophenyl)propanoic acid | ||
| SMILES | CC1=C(C=CC(=C1)C(C)(C(=O)O)N)P(=O)(O)O | ||
| Standard InChIKey | LLEOLNUKLKCAFG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H14NO5P/c1-6-5-7(10(2,11)9(12)13)3-4-8(6)17(14,15)16/h3-5H,11H2,1-2H3,(H,12,13)(H2,14,15,16) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A selective group III mGlu receptor antagonist (apparent Kd values are 5.1 and 488 μM for group III and group II mGlu receptors respectively; IC50 > 1 mM for group I, NMDA, AMPA and kainate receptors). |

UBP1112 Dilution Calculator

UBP1112 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.858 mL | 19.2901 mL | 38.5802 mL | 77.1605 mL | 96.4506 mL |
| 5 mM | 0.7716 mL | 3.858 mL | 7.716 mL | 15.4321 mL | 19.2901 mL |
| 10 mM | 0.3858 mL | 1.929 mL | 3.858 mL | 7.716 mL | 9.6451 mL |
| 50 mM | 0.0772 mL | 0.3858 mL | 0.7716 mL | 1.5432 mL | 1.929 mL |
| 100 mM | 0.0386 mL | 0.1929 mL | 0.3858 mL | 0.7716 mL | 0.9645 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Boc-Ile-Osu
Catalog No.:BCC2604
CAS No.:3392-08-3
- Boc-Gly-OSu
Catalog No.:BCC3397
CAS No.:3392-07-2
- Boc-Ala-OSu
Catalog No.:BCC3048
CAS No.:3392-05-0
- 2,3,4'-Trihydroxy-3',5'-dimethoxypropiophenone
Catalog No.:BCN1455
CAS No.:33900-74-2
- Vandetanib trifluoroacetate
Catalog No.:BCC2029
CAS No.:338992-53-3
- Mdivi 1
Catalog No.:BCC2402
CAS No.:338967-87-6
- Pabulenol
Catalog No.:BCC8347
CAS No.:33889-70-2
- Silychristin
Catalog No.:BCN2389
CAS No.:33889-69-9
- Demethyl tetrandrine
Catalog No.:BCN2624
CAS No.:33889-68-8
- LY 456236 hydrochloride
Catalog No.:BCC7347
CAS No.:338736-46-2
- CITCO
Catalog No.:BCC7749
CAS No.:338404-52-7
- Di-O-methylbergenin
Catalog No.:BCN5263
CAS No.:33815-57-5
- Anamorelin Fumarate
Catalog No.:BCC1363
CAS No.:339539-92-3
- Thalrugosidine
Catalog No.:BCN7785
CAS No.:33954-34-6
- H-D-Phe(4-Cl)-OMe.HCl
Catalog No.:BCC3174
CAS No.:33965-47-8
- Z-Leu-Osu
Catalog No.:BCC2592
CAS No.:3397-35-1
- Triacetonamine hydrochloride
Catalog No.:BCN5264
CAS No.:33973-59-0
- Pelargonidin-3-O-rutinosde chloride
Catalog No.:BCN3112
CAS No.:33978-17-5
- Clivorine
Catalog No.:BCN2067
CAS No.:33979-15-6
- 7-Oxodehydroabietinol
Catalog No.:BCN5265
CAS No.:33980-71-1
- Z-Ala-OSu
Catalog No.:BCC3057
CAS No.:3401-36-3
- Vinorine
Catalog No.:BCN4649
CAS No.:34020-07-0
- 1-BCP
Catalog No.:BCC6909
CAS No.:34023-62-6
- H-D-Phe-OtBu.HCl
Catalog No.:BCC3014
CAS No.:3403-25-6
Allosteric and Orthosteric Activators of mGluR8 Differentially Affect the Chemotherapeutic-Induced Human Neuroblastoma SH-SY5Y Cell Damage: The Impact of Cell Differentiation State.[Pubmed:29753314]
Basic Clin Pharmacol Toxicol. 2018 Oct;123(4):443-451.
The participation of group III metabotropic glutamate receptors (mGluRs) in cancer growth and progression is still an understudied issue. Based on our recent data on high expression of mGluR8 in human neuroblastoma SH-SY5Y cells, in this study, we evaluated the effect of an mGluR8-specific positive allosteric modulator (PAM: AZ12216052) and orthosteric agonist [(S)-3,4-DCPG ((S)-3,4-dicarboxyphenylglycine)] on chemotherapeutic (doxorubicin, irinotecan or cisplatin)-evoked cell damage in undifferentiated (UN-) and retinoic acid-differentiated (RA-) SH-SY5Y cells. The data showed that AZ12216052 as well as a group III mGluR antagonist (UBP1112) but not (S)-3,4-DCPG partially inhibited the cell damage evoked by doxorubicin, irinotecan or cisplatin in UN-SH-SY5Y cells. In RA-SH-SY5Y, we observed only a modest protective effect of mGluR8 PAM. In contrast, both types of mGluR8 activators significantly enhanced toxic effects of doxorubicin and irinotecan in RA-SH-SY5Y cells. These data suggest that in undifferentiated neuroblastoma malignant cells, some mGluR8 modulators can decrease cytotoxic effects of chemotherapeutics which exclude them from the group of putative anticancer agents. On the other hand, in SH-SY5Y cells differentiated to a more mature neuron-like phenotype, that is non-malignant cells, the mGluR8 activators can aggravate the chemotherapeutic neurotoxicity which is a well-known undesired effect of these drugs. Our pharmacological data add new observations to the unexplored field of research on the role of mGluR8 in cancer, pointing to complexity of response which could be mediated by particular types of mGluR8 ligands at least in neuroblastoma cells.
Modulatory function of NMDA glutamate receptor on MC3/MC4 receptors agonist-induced hypophagia in neonatal meat-type chicken.[Pubmed:28589422]
Vet Res Commun. 2017 Dec;41(4):241-248.
Melanocortin 3 and 4 receptors (MC3R and MC4R) are known as the main receptors for melanocortin-induced hypophagia in mammalian and poultry. Also, central glutamatergic system has mediatory role on function of the melanocortin system in some brain areas. So, the aim of the current study was to determine the role of MC3/MC4 receptors agonist on food intake and its interaction with glutamatergic in 3-h food-deprived (FD3) neonatal broilers. In experiment 1, chickens were intracerebroventricular (ICV) injected with control solution, MTII (MC3/MC4 receptors agonist; 2.45, 4.8 and 9.8 pmol). In experiment 2, control solution, SHU9119 (MC3/MC4 receptors antagonist; 0.5, 1 and 2 nmol) were ICV injected. In experiment 3, birds ICV injected with control solution, SHU9119 (0.5 nmol), MTII (9.8 pmol) and co-injection of the SHU9119 + MTII. Experiments 4-8 were similar to experiment 3, except birds injected with MK-801 (NMDA glutamate receptors antagonist, 15 nmol), CNQX (AMPA glutamate receptors antagonist; 390 nmol), AIDA (mGLUR1 glutamate receptors antagonist; 2 nmol), LY341495 (mGLUR2 glutamate receptors antagonist; 150 nmol) and UBP1112 (mGLUR3 glutamate receptors antagonist; 2 nmol) instead of SHU9119. Then, cumulative food intake was recorded until 120 min after injection. According to the results, dose dependent hypophagia observed after ICV injection of the MTII (p < 0.05). ICV injection of SHU9119 significantly increased food intake in birds (p < 0.05). Co-injection of SHU9119 + MTII significantly inhibited MTII- induced hypophagia in neonatal chicks (p < 0.05). In addition, hypophagia- induced by MTII was significantly attenuated with co-injection of MTII + MK-801(p < 0.05). These results suggested MC3 and MC4 receptors have inhibitory role on food intake and this effect is probably mediated by NMDA glutamate receptors in neonatal chickens.
Cannabinoid-glutamate interactions in the regulation of food intake in neonatal layer- type chicks: role of glutamate NMDA and AMPA receptors.[Pubmed:27000110]
Vet Res Commun. 2016 Jun;40(2):63-71.
The involvement of the endocannabinoid system in the brain functions is likely the conclusion of its capability to interact with specific neurotransmitters in several brain regions. The present study was designed to examine the role of the glutamatergic system on cannabinoid-induced hyperphagia in chicken. In this survey 10 experiments designed to investigate interaction of cannabinoidergic and glutamatergic systems on feeding behavior in neonatal chickens. In experiment 1, chicken were intracerebroventricular (ICV) injected with saline, 2-AG (2-Arachidonoylglycerol, 5.28 nmol, CB1 receptors agonist), MK-801(NMDA receptor antagonist, 15 nmol) and co-administration of 2-AG + MK-801. In experiment 2, injection of saline, 2-AG (5.28 nmol), CNQX) AMPA/kainate receptor antagonist, 390 nmol) and their combination (2-AG + CNQX) was done. In Experiment 3, injections were saline, 2-AG (5.28 nmol), AIDA)mGluR1 antagonist, 2 nmol) and 2-AG + AIDA. Experiments 4 and 5 were similar to experiment 3, except birds injected with LY341495 (mGLUR2 glutamate antagonist, 150 nmol) and UBP1112 (mGLUR3 glutamate antagonist, 2 nmol) instead of AIDA. Experiments 6-10 followed the procedure similar to experiments 1-5, except chickens received ICV injection of CB65 (CB2 receptor agonist, 3 nmol), instead of 2-AG. Then the cumulative food intake measured until 120 min post injection. According to the results, ICV injection of 2-AG and CB65 significantly increased food intake (P < 0.001). Co-injection of 2-AG and MK-801 significantly amplified hyperphagic effect of CB1 receptors agonist(P < 0.001). Moreover, co-administration of CB65 plus CNQX significantly increased CB65- induced hyperphagia in FD3 neonatal layer-type chickens (P < 0.001). These results suggest there is an interaction between endocannabinoids and glutamatergic systems via NMDA and AMPA receptors in feeding behavior of neonatal layer-type chickens.
The role of glutamatergic and GABAergic systems on serotonin- induced feeding behavior in chicken.[Pubmed:24006087]
Vet Res Commun. 2013 Dec;37(4):303-10.
It has been reported that serotonin can modulate glutamate and GABA release in central nervous system (CNS). The present study was designed to examine the role of glutamatergic and GABAergic systems on serotonin- induced feeding behavior in chickens. In Experiment 1 intracerebroventricular (ICV) injection of MK- 801(NMDA receptor antagonist, 15 nmol) performed followed by serotonin (10 mug). In experiments 2, 3, 4, 5, 6 and 7 prior to serotonin injection, chickens received CNQX (AMPA/kainate receptor antagonist, 390 nmol), AIDA (mGluR1 antagonist, 2 nmol), LY341495 (mGluR2 antagonist, 150 nmol), UBP1112 (mGluR3 antagonist, 2 nmol), picrotoxin (GABA A receptor antagonist, 0.5 mug), CGP54626 (GABAB receptor antagonist, 20 ng) respectively. Cumulative food intake was determined at 3 h post injection. The results of this study showed that the hypophagic effect of serotonin was significantly attenuated by pretreatment with MK- 801 and CNQX (p < 0.05) but AIDA, LY341495 and UBP1112 had no effect (p > 0.05). Also, the inhibitory effect of serotonin on food intake was amplified by picrotoxin (p < 0.05) while CGP54626 had no effect (p > 0.05). These results suggest that serotonin as a modulator probably interacts with glutamatergic (via NMDA and AMPA/Kainate receptors) and GABAergic (via GABAA receptor) systems on feeding behavior in chicken.
Group III metabotropic glutamate receptors and transient receptor potential vanilloid 1 co-localize and interact on nociceptors.[Pubmed:22609935]
Neuroscience. 2012 Aug 16;217:130-9.
Several lines of evidence indicate group III metabotropic glutamate receptors (mGluRs) have systemic anti-hyperalgesic effects. We hypothesized this could occur through modulation of transient receptor potential vanilloid 1 (TRPV1) receptors on nociceptors. To address this question we performed anatomical studies to determine if group III mGluRs were expressed on cutaneous axons and if they co-localized with TRPV1. Immunostaining at the electron microscopic level demonstrated that 22% of unmyelinated axons labeled for mGluR8. Immunostaining at the light microscopic level in lumbar dorsal root ganglia (DRG) demonstrated that 80% and 28% of neurons labeled for mGluR8 or TRPV1, respectively. Of those neurons labeled for mGluR8, 25% labeled for TRPV1; of those labeled for TRPV1, 71% labeled for mGluR8. In behavior studies intraplantar injection of the group III mGluR agonist, L-(+)-2-amino-4-phosphonobutyric acid (L-AP-4: 0.1, 1.0, and 10.0 muM) had no effect on paw withdrawal latency (PWL) to heat in naive rats but administration of 10 muM L-AP-4 prior to 0.05% capsaicin (CAP), significantly attenuated CAP-induced lifting/licking and reduced flinching behavior. The L-AP-4 effect was specific since administration of a group III antagonist alpha-methyl-3-methyl-4-phosphonophenylglycine (UBP1112) (100muM) blocked the L-AP-4 effect on CAP, resulting in behaviors similar to CAP alone. Intraplantar injection of UBP1112 alone did not result in nociceptive behaviors, indicating group III mGluRs are not tonically active. Finally, the anti-hyperalgesic effect of group III in this paradigm was local and not systemic since intraplantar administration of L-AP-4 in one hind paw did not attenuate nociceptive behaviors following CAP injection in the contralateral hind paw. Adenyl cyclase/cyclic AMP/PKA may be the second messenger pathway linking these two receptor families because intraplantar injection of forskolin (FSK, 10 muM) reduced PWL to heat and L-AP-4 reversed this FSK effect. Taken together, these results suggest group III mGluRs can negatively modulate TRPV1 through inhibition of adenyl cyclase and downstream intracellular activity, blocking TRPV1-induced activation of nociceptors.
Group II/III metabotropic glutamate receptors exert endogenous activity-dependent modulation of TRPV1 receptors on peripheral nociceptors.[Pubmed:21900552]
J Neurosci. 2011 Sep 7;31(36):12727-37.
There is pharmacological evidence that group II and III metabotropic glutamate receptors (mGluRs) function as activity-dependent autoreceptors, inhibiting transmission in supraspinal sites. These receptors are expressed by peripheral nociceptors. We investigated whether mGluRs function as activity-dependent autoreceptors inhibiting pain transmission to the rat CNS, particularly transient receptor potential vanilloid 1 (TRPV1)-induced activity. Blocking peripheral mGluR activity by intraplantar injection of antagonists LY341495 [(2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid] (LY) (20, 100 mum, group II/III), APICA [(RS)-1-amino-5-phosphonoindan-1-carboxylic acid] (100 mum, group II), or UBP1112 (alpha-methyl-3-methyl-4-phosphonophenylglycine) (30 mum, group III) increased capsaicin (CAP)-induced nociceptive behaviors and nociceptor activity. In contrast, group II agonist APDC [(2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate] (0.1 mum) or group III agonist l-(+)-2-amino-4-phosphonobutyric acid (l-AP-4) (10 mum) blocked the LY-induced increase. Ca(2+) imaging in dorsal root ganglion (DRG) cells confirmed LY enhanced CAP-induced Ca(2+) mobilization, which was blocked by APDC and l-AP-4. We hypothesized that excess glutamate (GLU) released by high intensity and/or prolonged stimulation endogenously activated group II/III, dampening nociceptor activation. In support of this, intraplantar GLU + LY produced heat hyperalgesia, and exogenous GLU + LY applied to nociceptors produced enhanced nociceptor activity and thermal sensitization. Intraplantar Formalin, known to elevate extracellular GLU, enhanced pain behaviors in the presence of LY. LY alone produced no pain behaviors, no change in nociceptor discharge rate or heat-evoked responses, and no change in cytosolic Ca(2+) in DRG cells, demonstrating a lack of tonic inhibitory control. Group II/III mGluRs maintain an activity-dependent autoinhibition, capable of significantly reducing TRPV1-induced activity. They are endogenously activated after high-frequency and/or prolonged nociceptor stimulation, acting as built-in negative modulators of TRPV1 and nociceptor function, reducing pain transmission to the CNS.
Differential changes of group II and group III mGluR function in central amygdala neurons in a model of arthritic pain.[Pubmed:16760343]
J Neurophysiol. 2006 Oct;96(4):1803-15.
Metabotropic glutamate receptors (mGluRs) play important roles in neuroplasticity and disorders such as persistent pain. Group I mGluRs contribute to pain-related sensitization and synaptic plasticity of neurons in the laterocapsular division of the central nucleus of the amygdala (CeLC), although the roles of groups II and III mGluRs are not known. Extracellular single-unit recordings were made from 60 CeLC neurons in anesthetized adult rats. Background activity and evoked responses were measured before and during the development of the kaolin/carrageenan-induced knee-joint arthritis. Drugs were administered into the CeLC by microdialysis before and/or after arthritis induction. A selective group III mGluR agonist (LAP4) inhibited CeLC neurons' responses to stimulation of the knee and ankle in arthritis (n = 7) more potently than under normal conditions (n = 14). A selective group II agonist (LY354740) inhibited responses under normal conditions (n = 12) and became more potent in inhibiting responses to noxious stimulation of the knee in arthritis (n = 10). The effect of LY354740 on innocuous stimulation of the knee and stimulation of the ankle did not change in arthritis. Antagonists for groups II (EGLU, n = 9) and III (UBP1112, n = 8) had no effects under normal conditions. In arthritis, UPB1112 (n = 5) facilitated the responses to stimulation of knee and ankle, whereas EGLU (n = 5) selectively increased the responses to stimulation of the knee. These data suggest that mGluRs of groups II and III can inhibit nociceptive processing in CeLC neurons. The increased function and endogenous activation of group II mGluRs in the arthritis pain model appear more input-selective than the general changes of group III mGluRs.
Modulation of excitatory synaptic transmission by endogenous glutamate acting on presynaptic group II mGluRs in rat substantia nigra compacta.[Pubmed:16273546]
J Neurosci Res. 2005 Dec 15;82(6):778-87.
Excitatory synaptic inputs from the subthalamic nucleus (STN) have been proposed to underlie burst firing of substantia nigra pars compacta (SNc) dopamine (DA) neurons in Parkinson's disease. Given the potential importance of the STN-SNc synapse in health and disease, our goal was to study how transmission at this synapse is regulated. We tested the hypothesis that neurotransmission at STN-SNc synapses is tonically inhibited by endogenous glutamate acting on presynaptic group II metabotropic glutamate receptors (mGluRs). By using whole-cell recording techniques in brain slices, we examined the effect of LY341495, a mGluR antagonist that is most potent at group II mGluRs, on excitatory postsynaptic currents (EPSCs) that either were evoked in SNc DA neurons by stimulation of the STN or were spontaneously occurred in the presence of tetrodotoxin (miniature EPSCs; mEPSCs). LY341495 increased the evoked EPSC amplitude and mEPSC frequency without changing mEPSC amplitude. In contrast, the group III mGluR antagonist UBP1112 failed to increase the evoked EPSC amplitude. An elevation of extracellular glutamate concentration by a glutamate transporter inhibitor, TBOA, suppressed the evoked EPSCs. LY341495, but not UBP1112, partially reversed the TBOA action. The modulations of EPSCs by TBOA and LY341495 were associated with changes in paired-pulse facilitation ratio. Furthermore, TBOA decreased mEPSC frequency, which was partially reversed by LY341495, without affecting mEPSC amplitude. The results indicate that presynaptic group II mGluRs at STN-SNc synapses appear to be partially activated by a basal level of extracellular glutamate and able to sense the change in extracellular glutamate concentration, subsequently modulating synaptic glutamate release.
Differential roles of mGlu8 receptors in the regulation of glutamate and gamma-aminobutyric acid release at periaqueductal grey level.[Pubmed:16084932]
Neuropharmacology. 2005;49 Suppl 1:157-66.
We investigated the role of group III metabotropic glutamate (mGlu) receptors on glutamate and GABA releases at the periaqueductal grey (PAG) level by using in vivo microdialysis in rats. Intra-PAG perfusion of either L-(+)-2-amino-4-phosphonobutyric acid (L-AP4, 100-300 microM), (RS)-4-phosphonophenylglycine ((RS)-PPG, 100-300 microM) selective agonists of group III mGlu receptors, or (S)-3,4-dicarboxyphenylglycine ((S)-3,4-DCPG, 50-100 microM), a selective agonist of mGlu8 receptor, increased glutamate and decreased GABA extracellular concentrations. (RS)-alpha-methylserine-O-phosphate (MSOP, 0.5 mM), a selective group III receptor antagonist, perfused in combination with (S)-3,4-DCPG, L-AP4 or (RS)-PPG, antagonised the effects induced by these agonists on both extracellular glutamate and GABA values. alpha-Methyl-3-methyl-4-phosphonophenylglycine (UBP1112, 300 microM), a group III mGlu receptor antagonist, perfused in combination with (RS)-PPG or (S)-3,4-DCPG, antagonised the effects induced by these agonists. Intra-PAG perfusion with forskolin (100 microM), an activator of adenylate cyclase, increased dialysate glutamate and GABA levels. Moreover, intra-PAG perfusion with N-[2-(p-bromocinnamyl-amino)ethyl]-5-isoquinolinesulfonamide dihydrochloride (H-89) (100 microM), a protein kinase (PKA) inhibitor, abolished the effect of (S)-3,4-DCPG on both glutamate and GABA releases. H-89, per se, did not modify glutamate release but reduced extracellular GABA value at the higher dosage used (200 microM). These data suggest that group III mGlu receptors in the PAG modulate the releases of glutamate and GABA conversely. In particular, both the facilitation of glutamate and the inhibition of GABA releases require the participation of coupling to adenylate cyclase and the subsequent activation of the PKA pathway.
Enhanced group III mGluR-mediated inhibition of pain-related synaptic plasticity in the amygdala.[Pubmed:15081788]
Neuropharmacology. 2004 Jun;46(7):918-26.
Pain has a strong emotional component. A key player in emotionality, the amygdala is also involved in pain processing. Our previous studies showed synaptic plasticity in the central nucleus of the amygdala (CeA) in a model of arthritic pain. Here, we address the role of group III metabotropic glutamate receptors (mGluRs) in the regulation of synaptic transmission in CeA neurons. Whole-cell current- and voltage-clamp recordings were made from neurons in the latero-capsular part of the CeA in brain slices from control rats and arthritic rats (>6 h postinduction). The latero-capsular part of the CeA is the target of the spino-parabrachio-amygdaloid pain pathway and is now designated as the "nociceptive amygdala". Monosynaptic excitatory postsynaptic currents (EPSCs) were evoked by electrical stimulation of afferents from the pontine parabrachial (PB) area. LAP4 decreased the amplitude of EPSCs more potently in CeA neurons from arthritic rats (EC(50)=1.2 nM) than in control animals (EC(50)=11.5 nM). The inhibitory effect of LAP4 was reversed by a selective group III mGluR antagonist (UBP1112). During the application of LAP4, paired-pulse facilitation was increased, while no significant changes in slope conductance and action potential firing rate of CeA neurons were observed. These data suggest that presynaptic group III mGluRs are involved in the regulation of synaptic plasticity in the amygdala in an arthritis pain model.
Phenylglycine derivatives as antagonists of group III metabotropic glutamate receptors expressed on neonatal rat primary afferent terminals.[Pubmed:12922940]
Br J Pharmacol. 2003 Aug;139(8):1523-31.
1. Three novel phenylglycine analogues; (RS)-alpha-methyl-3-chloro-4-phosphonophenylglycine (UBP1110), (RS)-alpha-methyl-3-methoxy-4-phosphonophenylglycine (UBP1111) and (RS)-alpha-methyl-3-methyl-4-phosphonophenylglycine (UBP1112) antagonised the depression of the fast component of the dorsal root-evoked ventral root potential induced by (S)-AP4 with apparent K(D) values of: 7.4+/-2.3, 5.4+/-0.6 and 5.1+/-0.3 micro M (all n=3), respectively. 2. A Schild analysis of the antagonism of (S)-AP4 induced depression of synaptic transmission by UBP1112 revealed a pA(2) value of 5.3 and a slope of 0.81+/-0.26 (n=9). 3. None of the phenylglycines tested were potent antagonists of responses mediated by group II mGlu receptors (apparent K(D) values >480 micro M). UBP1112 when tested at a concentration of 1 mM had little or no activity on (S)-3,5-DHPG-, NMDA-, AMPA- or kainate-induced responses on motoneurones. 4. UBP1110, UBP1111 and UBP1112 are at least 100-fold selective for group III over group I and II mGlu receptors expressed in the spinal cord making them the most potent, selective, antagonists yet tested at (S)-AP4 sensitive receptors in the spinal cord.
Synthesis of phenylglycine derivatives as potent and selective antagonists of group III metabotropic glutamate receptors.[Pubmed:11277518]
Bioorg Med Chem Lett. 2001 Mar 26;11(6):777-80.
The syntheses of a range of ring and alpha-substituted 4-phosphonophenylglycines are described. A brief discussion of the antagonist activities of compounds 4-10 on group I, II and III metabotropic glutamate (mGlu) receptors expressed in the neonatal rat spinal cord is included.


