PachypodolCAS# 33708-72-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 33708-72-4 | SDF | Download SDF |
| PubChem ID | 5281677 | Appearance | White-yellowish powder |
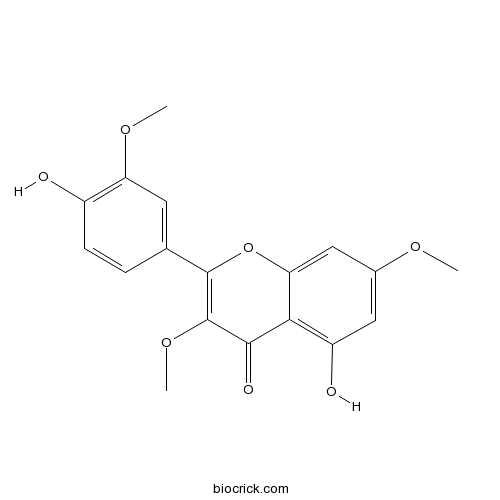
| Formula | C18H16O7 | M.Wt | 344.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 4',5-Dihydroxy 3,3',7-trimethoxyflavone; Isorhamnetin 3,7-dimethyl ether; Quercetin 3,3',7-trimethyl ether; 3,3',7-Trimethylquercetin | ||
| Solubility | Soluble in methanol; slightly soluble in water | ||
| Chemical Name | 5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,7-dimethoxychromen-4-one | ||
| SMILES | COC1=CC(=C2C(=C1)OC(=C(C2=O)OC)C3=CC(=C(C=C3)O)OC)O | ||
| Standard InChIKey | KQFUXLQBMQGNRT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H16O7/c1-22-10-7-12(20)15-14(8-10)25-17(18(24-3)16(15)21)9-4-5-11(19)13(6-9)23-2/h4-8,19-20H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Pachypodol has cytotoxic potential ,can inhibit the growth of CaCo 2 colon cancer cell line in vitro. 2. Pachypodol has antibacterial and antifungal activities against Bacillus subtilis, Staphylococcus aureus, Staphylococcus faecalis, Echerichia coli, Pseudomonas aeruginosa, Candida albicans, Candida krusei and Candida galabrata. 3. Pachypodol exhibits anti-emetic effects. 4. Pachypodol inhibits the water-splitting enzyme activity. |
| Targets | Antifection |

Pachypodol Dilution Calculator

Pachypodol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9044 mL | 14.5222 mL | 29.0444 mL | 58.0889 mL | 72.6111 mL |
| 5 mM | 0.5809 mL | 2.9044 mL | 5.8089 mL | 11.6178 mL | 14.5222 mL |
| 10 mM | 0.2904 mL | 1.4522 mL | 2.9044 mL | 5.8089 mL | 7.2611 mL |
| 50 mM | 0.0581 mL | 0.2904 mL | 0.5809 mL | 1.1618 mL | 1.4522 mL |
| 100 mM | 0.029 mL | 0.1452 mL | 0.2904 mL | 0.5809 mL | 0.7261 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 19,20-(E)-Vallesamine
Catalog No.:BCN5260
CAS No.:3368-87-4
- Hypophyllanthin
Catalog No.:BCN5259
CAS No.:33676-00-5
- Secnidazole
Catalog No.:BCC4971
CAS No.:3366-95-8
- H-Thr-Obzl.HCl
Catalog No.:BCC2674
CAS No.:33645-24-8
- (S)-(+)-Ketamine hydrochloride
Catalog No.:BCC7930
CAS No.:33643-47-9
- 1-O-Acetylbritannilactone
Catalog No.:BCN7715
CAS No.:33627-41-7
- Britannin
Catalog No.:BCN2366
CAS No.:33627-28-0
- Desoxyrhapontigenin
Catalog No.:BCN6479
CAS No.:33626-08-3
- Britannilactone
Catalog No.:BCN3509
CAS No.:33620-72-3
- Ispinesib (SB-715992)
Catalog No.:BCC2509
CAS No.:336113-53-2
- Myricanol
Catalog No.:BCN5258
CAS No.:33606-81-4
- Polpunonic acid
Catalog No.:BCN7136
CAS No.:33600-93-0
- (-)-Gallocatechin
Catalog No.:BCN5927
CAS No.:3371-27-5
- Voacamine
Catalog No.:BCN8433
CAS No.:3371-85-5
- 4,4'-Di-O-methylellagic acid
Catalog No.:BCN3709
CAS No.:3374-77-4
- Cannabivarin
Catalog No.:BCN7587
CAS No.:33745-21-0
- Abieta-8,11,13-triene-7,15,18-triol
Catalog No.:BCN5262
CAS No.:337527-10-3
- Androstanolone heptanoate
Catalog No.:BCC8827
CAS No.:33776-88-4
- Hederacholchiside E
Catalog No.:BCC8329
CAS No.:33783-82-3
- Deodarin
Catalog No.:BCN6874
CAS No.:33788-39-5
- H-D-Abu-OH
Catalog No.:BCC3202
CAS No.:338-69-2
- Methasterone
Catalog No.:BCC9027
CAS No.:3381-88-2
- Di-O-methylbergenin
Catalog No.:BCN5263
CAS No.:33815-57-5
- CITCO
Catalog No.:BCC7749
CAS No.:338404-52-7
Pachypodol, a flavonol from the leaves of Calycopteris floribunda, inhibits the growth of CaCo 2 colon cancer cell line in vitro.[Pubmed:18570232]
Phytother Res. 2008 Dec;22(12):1684-7.
Calycopteris floribunda Lam., commonly known as 'goichia lata or goache lata', is a large climbing woody shrub from Bangladesh, and well distributed in a number of other south-east Asian countries. Traditionally, C. floribunda has been used in colic, as an antihelminthic, astringent and carminative, and for the treatment of diarrhoea, dysentery, jaundice and malaria in many countries including Bangladesh. Pachypodol (5,4'-dihydroxy-3,7,3'-trimethoxyflavone) has been isolated from the leaves of C. floribunda by repeated column chromatography on silica gel, and the structure confirmed by spectroscopic means. While the general toxicity of Pachypodol was determined by the brine shrimp lethality assay, the cytotoxic potential of this flavonoid has been evaluated by the Promega's CellTiter 96 Non-Radioactive Cell Proliferation Assay using the CaCo-2 colon cancer cell line (IC(50) = 185.6 microM). A summary of the biological activities of Pachypodol reported to date is also presented.
Phosphatidylinositol 4-kinase III beta is the target of oxoglaucine and pachypodol (Ro 09-0179) for their anti-poliovirus activities, and is located at upstream of the target step of brefeldin A.[Pubmed:25891300]
Microbiol Immunol. 2015 Jun;59(6):338-47.
In recent years, phosphatidylinositol 4-kinase III beta (PI4KB) has emerged as a conserved target of anti-picornavirus compounds. In the present study, PI4KB was identified as the direct target of the plant-derived anti-picornavirus compounds, oxoglaucine and Pachypodol (also known as Ro 09-0179). PI4KB was also identified as the target via which Pachypodol interferes with brefeldin A (BFA)-induced Golgi disassembly in non-infected cells. Oxysterol-binding protein (OSBP) inhibitor also has interfering activity against BFA. It seems that this interference is not essential for the anti-poliovirus (PV) activities of BFA and PI4KB/OSBP inhibitors. BFA inhibited early to late phase PV replication (0 to 6 hr postinfection) as well as PI4KB inhibitor, but with some delay compared to guanidine hydrochloride treatment. In contrast with PI4KB/OSBP inhibitors, BFA inhibited viral nascent RNA synthesis, suggesting that BFA targets some step of viral RNA synthesis located downstream of the PI4KB/OSBP pathway in PV replication. Our results suggest that PI4KB is a major target of anti-picornavirus compounds identified in vitro for their anti-picornavirus activities and for some uncharacterized biological phenomena caused by these compounds, and that BFA and PI4KB/OSBP inhibitors synergistically repress PV replication by targeting distinct steps in viral RNA replication.
Pachypodol from Croton ciliatoglanduliferus Ort. as water-splitting enzyme inhibitor on thylakoids.[Pubmed:16478239]
J Agric Food Chem. 2006 Feb 22;54(4):1217-21.
A bioactivity-guided chemical study of aerial parts of Croton ciliatoglanduliferus Ort. led to the isolation for the first time of the flavonoids retusin (5-hydroxy-3,7,3',4'-tetramethoxyflavone) (1) and Pachypodol (5,4'-dihydroxy-3,7,3'-trimethoxyflavone) (2) from the n-hexane extract. Compounds 1 and 2 were separated by preparative thin-layer chromatography. Compound 2 was the most active compound on ATP synthesis inhibition. The I50 value was 51 microM. Pachypodol behaves as a Hill reaction inhibitor. It inhibited the uncoupled electron flow on photosystem II partial reaction from water to dichlorophenol indophenol (DCPIP) and from water to sodium silicomolybdate. However, the uncoupled partial reaction from diphenylcarbazide to DCPIP and the uncoupled photosystem I from DCPIPred to MV were not inhibited by 2. These results were corroborated by fluorescence decay data. Therefore, Pachypodol inhibits the water-splitting enzyme activity. Compound 1 with a 4'-methoxy group was a weak inhibitor, indicating that the 4' free -OH group is important for strong inhibition.
Anti-emetic principles of Pogostemon cablin (Blanco) Benth.[Pubmed:10374246]
Phytomedicine. 1999 May;6(2):89-93.
Bioassay-guided fractionation of anti-emetic extracts and constituents of 8 traditional Chinese herbal drugs was performed. Twenty extracts described in Table 1 showed anti-emetic activity on copper sulfate induced-emesis in young chicks. From the n-hexane extract of Pogostemon cablin, patchouli alcohol (1), pogostol (2), stigmast-4-en-3-one (3), retusin (4), and Pachypodol (5) were tested and exhibited anti-emetic effects.


