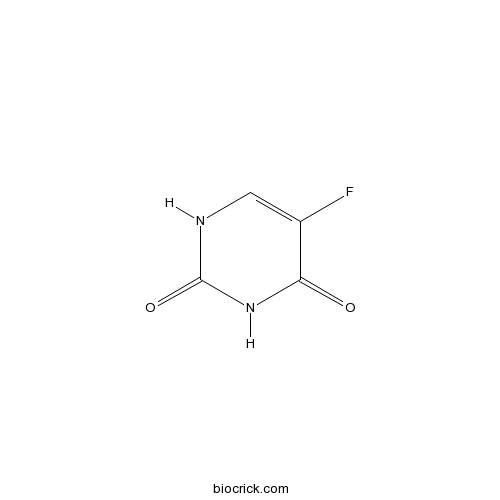
Fluorouracil (Adrucil)Antitumor agent;inhibitor of thymidylate synthase CAS# 51-21-8 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 51-21-8 | SDF | Download SDF |
| PubChem ID | 3385 | Appearance | Powder |
| Formula | C4H3FN2O2 | M.Wt | 130.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 5-FU | ||
| Solubility | H2O : 16.67 mg/mL (128.15 mM; Need ultrasonic) DMSO : 15 mg/mL (115.31 mM; Need ultrasonic and warming) | ||
| Chemical Name | 5-fluoro-1H-pyrimidine-2,4-dione | ||
| SMILES | C1=C(C(=O)NC(=O)N1)F | ||
| Standard InChIKey | GHASVSINZRGABV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C4H3FN2O2/c5-2-1-6-4(9)7-3(2)8/h1H,(H2,6,7,8,9) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Anticancer agent. Metabolized to form fluorodeoxyuridine monophosphate (FdUMP), fluorodeoxyuridine triphosphate (FdUTP) and fluorouridine (FUTP). FdUMP inhibits thymidylate synthase, causing a reduction in dTMP synthesis. FUTP and FdUTP are misincorporated into RNA and DNA respectively. |

Fluorouracil (Adrucil) Dilution Calculator

Fluorouracil (Adrucil) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.6864 mL | 38.432 mL | 76.864 mL | 153.7279 mL | 192.1599 mL |
| 5 mM | 1.5373 mL | 7.6864 mL | 15.3728 mL | 30.7456 mL | 38.432 mL |
| 10 mM | 0.7686 mL | 3.8432 mL | 7.6864 mL | 15.3728 mL | 19.216 mL |
| 50 mM | 0.1537 mL | 0.7686 mL | 1.5373 mL | 3.0746 mL | 3.8432 mL |
| 100 mM | 0.0769 mL | 0.3843 mL | 0.7686 mL | 1.5373 mL | 1.9216 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fluorouracil (Adrucil), a heterocyclic aromatic organic compound, is a potent anticancer agent widely used for the treatment of solid tumors, including breast cancer, ovarian cancer, head and neck cancer, and colon cancer. As an analogue of uracil, fluorouracil has a fluorine atom replacing the hydrogen atom at the C-5 position. Due to its similar chemical structure to DNA and RNA, fluorouracil and metabolites exert strong anticancer activities through incorporation into DNA and RNA and inhibition of thymidylate synthase (TS). Fluorouracil is metabolized into fluorodeoxyuridine monophosphate (FdUMP), which inhibits TS by forming a stable complex with it and subsequently suppresses the production of deoxythymidine monophosphate (dTMP), an essential enzyme involved in DNA replication and repair, leading to cytotoxicity and cell death.
Reference
Ning Zhang, Ying Yin, Shen-Jie Xu and Wei-Shan Chen. 5-Fluorouracil: mechanisms of resistance and reversal strategies. Molecules 2008, 13, 1551-1569
Michael D. Wyatt and David M. Wilson III. Participation of DNA repair in the response to 5-fluorouracil. Cell Mol Life Sci. 2009; 66(5): 788-799
- Benzimidazole
Catalog No.:BCC8847
CAS No.:51-17-2
- Procaine HCl
Catalog No.:BCC5072
CAS No.:51-05-8
- Pronethalol hydrochloride
Catalog No.:BCC5678
CAS No.:51-02-5
- 7ACC1
Catalog No.:BCC5553
CAS No.:50995-74-9
- 16-Methoxystrychnidin-10-One
Catalog No.:BCN8472
CAS No.:5096-72-0
- N-Methylcoclaurine
Catalog No.:BCN7079
CAS No.:5096-70-8
- Canadine
Catalog No.:BCN5626
CAS No.:5096-57-1
- Crotanecine
Catalog No.:BCN1963
CAS No.:5096-50-4
- Anacrotine
Catalog No.:BCN2057
CAS No.:5096-49-1
- Carminomycin
Catalog No.:BCC6379
CAS No.:50935-04-1, 39472-31-6
- Verminoside
Catalog No.:BCN5625
CAS No.:50932-19-9
- Mizoribine
Catalog No.:BCC4454
CAS No.:50924-49-7
- Tiratricol
Catalog No.:BCC4738
CAS No.:51-24-1
- Isoprenaline HCl
Catalog No.:BCC4328
CAS No.:51-30-9
- Scopolamine
Catalog No.:BCN5045
CAS No.:51-34-3
- H-Hyp-OH
Catalog No.:BCC3250
CAS No.:51-35-4
- Norepinephrine
Catalog No.:BCN2206
CAS No.:51-41-2
- Epinephrine Bitartrate
Catalog No.:BCC4348
CAS No.:51-42-3
- Adrenaline
Catalog No.:BCN2191
CAS No.:51-43-4
- Histamine
Catalog No.:BCN2188
CAS No.:51-45-6
- L-Thyroxine
Catalog No.:BCC4917
CAS No.:51-48-9
- Propylthiouracil
Catalog No.:BCC4931
CAS No.:51-52-5
- Atropine
Catalog No.:BCN5639
CAS No.:51-55-8
- Homatropine Bromide
Catalog No.:BCC4570
CAS No.:51-56-9
Randomized controlled trial of late-course concurrent versus sequential chemoradiotherapy after mastectomy and axillary surgery in locally advanced breast cancer.[Pubmed:29019894]
Medicine (Baltimore). 2017 Oct;96(41):e8252.
BACKGROUND: Concurrent chemoradiotherapy could increase the local control rate in patients with high recurrence risk after breast-conserving surgery, but the effect of concurrent chemoradiotherapy after mastectomy and axillary dissection is not clear. The aim of the study was to compare the effects of late-course concurrent chemoradiotherapy (CCRT) versus sequential therapy (SCRT) after mastectomy and axillary surgery in locally advanced breast cancer. METHODS: This was a randomized controlled trial of 155 patients with stage pT3-4p N1-3c M0 or pAnyT pN2-3c M0 breast cancer undergoing 5-fluorouracil+epirubicin+cyclophosphamide followed by docetaxel (FEC-D) chemotherapy after mastectomy and axillary dissection. Patients were randomized to the CCRT group (intensity-modulated radiation therapy was performed concurrently with docetaxel) or to the SCRT group (radiotherapy after chemotherapy). Recurrences, adverse reactions, and short-term effects were observed. RESULTS: All the patients completed the planned therapy. The median follow-up was 39 (range, 16-62) months. Compared with SCRT, the 3-year local-regional recurrence-free survival (LRFS) in the CCRT group was improved (81.8% vs 92.3%, P = .046). There was no significant difference in 3-year disease-free survival (DFS) and overall survival (OS). In the pT3-4 pN1-3 cM0 subgroup, the 3-year local recurrence-free survival and DFS were significantly improved in the CCRT group (69.4% vs 88.2%, P = .036; and 41.7% vs 72.6%, P = .049, respectively). No significant difference was observed adverse reactions between the 2 groups. CONCLUSION: LRFS of patients with locally advanced invasive breast cancer after mastectomy and axillary surgery was better with CCRT than with SCRT and with similar profiles of adverse reactions. The DFS of patients staged pT3-4 pN1-3 cM0 was also improved.
Activation of the p53 Transcriptional Program Sensitizes Cancer Cells to Cdk7 Inhibitors.[Pubmed:29020632]
Cell Rep. 2017 Oct 10;21(2):467-481.
Cdk7, the CDK-activating kinase and transcription factor IIH component, is a target of inhibitors that kill cancer cells by exploiting tumor-specific transcriptional dependencies. However, whereas selective inhibition of analog-sensitive (AS) Cdk7 in colon cancer-derived cells arrests division and disrupts transcription, it does not by itself trigger apoptosis efficiently. Here, we show that p53 activation by 5-fluorouracil or nutlin-3 synergizes with a reversible Cdk7(as) inhibitor to induce cell death. Synthetic lethality was recapitulated with covalent inhibitors of wild-type Cdk7, THZ1, or the more selective YKL-1-116. The effects were allele specific; a CDK7(as) mutation conferred both sensitivity to bulky adenine analogs and resistance to covalent inhibitors. Non-transformed colon epithelial cells were resistant to these combinations, as were cancer-derived cells with p53-inactivating mutations. Apoptosis was dependent on death receptor DR5, a p53 transcriptional target whose expression was refractory to Cdk7 inhibition. Therefore, p53 activation induces transcriptional dependency to sensitize cancer cells to Cdk7 inhibition.
A Comprehensive Review of Sequencing and Combination Strategies of Targeted Agents in Metastatic Colorectal Cancer.[Pubmed:29021377]
Oncologist. 2018 Jan;23(1):25-34.
The emergence of targeted therapies for the treatment of metastatic colorectal cancer (mCRC) has considerably improved survival, but has also resulted in a dilemma of identifying the optimal sequence and combination of various agents in the mCRC treatment landscape. A number of cytotoxic agents, including irinotecan, oxaliplatin, 5-fluorouracil, capecitabine, and TAS-102, are available for treatment of mCRC. Additionally, whereas patients harboring rat sarcoma viral oncogene homolog (RAS)-wild type mCRC can be treated with the anti-epidermal growth factor receptor antibodies cetuximab and panitumumab or antiangiogenic agents (bevacizumab, ziv-aflibercept, and ramucirumab), patients with RAS-mutant mCRC are limited to antiangiogenic agents as biologic options. Regorafenib, a multikinase inhibitor, can be used in both RAS subgroups. As such, the recommended sequence of therapies that should be received by each subgroup must also be considered separately. This review provides an overview of recent clinical data for approved and investigational targeted therapies that have been studied across different mCRC treatment lines and patient subgroups. It also examines emerging trends in the treatment landscape for mCRC, including treatment with immune checkpoint inhibitors and the utilization of genomic profiling. IMPLICATIONS FOR PRACTICE: Currently, there are no established guidelines for optimal sequencing of cytotoxic or targeted agents in metastatic colorectal cancer (mCRC). This review provides a snapshot of the current mCRC treatment paradigm and examines the latest clinical data that support the utilization of several targeted agents alone or in combination with backbone chemotherapy across different lines of treatment and patient populations, highlighting recommendations for their usage. Recent advances in the treatment landscape are also summarized, including genomic profiling and preliminary results with immune checkpoint inhibitors.
Lyotropic Liquid-Crystalline Nanosystems as Drug Delivery Agents for 5-Fluorouracil: Structure and Cytotoxicity.[Pubmed:29023126]
Langmuir. 2017 Oct 31;33(43):12369-12378.
Lyotropic cubic liquid-crystalline systems have received increasing attention due to their unique microstructural and physicochemical properties as efficient nanocarriers for drug delivery. We report the preparation and characterization of bulk phases and cubosome dispersions of phytantriol loaded with the anticancer drug 5-fluorouracil, in neutral and anionic forms. In both cases, a Pn3m cubic phase was observed. The phytantriol phase behavior can be influenced by the addition of ionic agents, and, to this purpose, a positively charged lipid, such as N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride salt (DOTAP), was included in the studied formulations. It was found to induce a variation of the spontaneous membrane curvature of the phytantriol lipid bilayer, generating a transition from the Pn3m to the Im3m cubic phase. When 5-fluorouracil, in its anionic form (5-FUs), was encapsulated in these latter systems, a further transition to the HII hexagonal phase was observed as a consequence of the formation of a complex phytantriol/DOTAP/5-FUs. The physicochemical characterization was performed with various complementary techniques including synchrotron small-angle X-ray scattering, dynamic light scattering, and attenuated total reflection Fourier transform infrared and UV resonance Raman spectroscopies. Encapsulation of 5-fluorouracil in the corresponding nanodispersions was evaluated, and their in vitro cytotoxicity was assessed in MDA-MB-231 cell line. Phytantriol cubosomes containing 5-fluorouracil showed a higher toxicity compared with the bare drug solution, and hence they represent potential nanocarriers in the delivery of 5-fluorouracil for cancer therapy.
Metastatic primary seminal vesicle adenocarcinoma: management of a rare tumour with multiagent chemotherapy and hormonal therapy.[Pubmed:29021144]
BMJ Case Rep. 2017 Oct 10;2017. pii: bcr-2017-221896.
Primary seminal vesicle adenocarcinoma is one of the rarest genitourinary cancers. The pathogenesis is unknown and clinical manifestations are protean. There is no defined treatment for this disease and various combinations of surgery, chemotherapy, radiation therapy and hormonal therapy have been used in the past. Here, we have reported a primary seminal vesicle adenocarcinoma with hepatic metastases, managed with multiagent chemotherapy (oxaliplatin and 5-fluorouracil based) and androgen ablation (with triptorelin). The key to management of such a case is early diagnosis and multimodal treatment. The reported survival rate continues to be poor even for a localised disease. A consolidated follow-up protocol ensures early diagnosis of recurrent or metastatic disease so that second-line therapy can be started.
5-fluorouracil: mechanisms of action and clinical strategies.[Pubmed:12724731]
Nat Rev Cancer. 2003 May;3(5):330-8.
5-fluorouracil (5-FU) is widely used in the treatment of cancer. Over the past 20 years, increased understanding of the mechanism of action of 5-FU has led to the development of strategies that increase its anticancer activity. Despite these advances, drug resistance remains a significant limitation to the clinical use of 5-FU. Emerging technologies, such as DNA microarray profiling, have the potential to identify novel genes that are involved in mediating resistance to 5-FU. Such target genes might prove to be therapeutically valuable as new targets for chemotherapy, or as predictive biomarkers of response to 5-FU-based chemotherapy.
Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism.[Pubmed:12084461]
Biochim Biophys Acta. 2002 Jul 18;1587(2-3):194-205.
Thymidylate synthase (TS) is a key enzyme in the de novo synthesis of 2'-deoxythymidine-5'-monophosphate (dTMP) from 2'-deoxyuridine-5'-monophosphate (dUMP), for which 5,10-methylene-tetrahydrofolate (CH(2)-THF) is the methyl donor. TS is an important target for chemotherapy; it is inhibited by folate and nucleotide analogs, such as by 5-fluoro-dUMP (FdUMP), the active metabolite of 5-fluorouracil (5FU). FdUMP forms a relatively stable ternary complex with TS and CH(2)THF, which is further stabilized by leucovorin (LV). 5FU treatment can induce TS expression, which might bypass dTMP depletion. An improved efficacy of 5FU might be achieved by increasing and prolonging TS inhibition, a prevention of dissociation of the ternary complex, and prevention of TS induction. In a panel of 17 colon cancer cells, including several variants with acquired resistance to 5FU, sensitivity was related to TS levels, but exclusion of the resistant variants abolished this relation. For antifolates, polyglutamylation was more important than the intrinsic TS level. Cells with low p53 levels were more sensitive to 5FU and the antifolate raltitrexed (RTX) than cells with high, mutated p53. Free TS protein down-regulates its own translation, but its transcription is regulated by E2F, a cell cycle checkpoint regulator. Together, this results in low TS levels in stationary phase cells. Although cells with a low TS might theoretically be more sensitive to 5FU, the low proliferation rate prevents induction of DNA damage and 5FU toxicity. TS levels were not related to polymorphisms of the TS promoter. Treatment with 5FU or RTX rapidly induced TS levels two- to five-fold. In animal models, 5FU treatment resulted in TS inhibition followed by a two- to three-fold TS induction. Both LV and a high dose of 5FU not only enhanced TS inhibition, but also prevented TS induction and increased the antitumor effect. In patients, TS levels as determined by enzyme activity assays, immunohistochemistry and mRNA expression, were related to a response to 5FU. 5FU treatment initially decreased TS levels, but this was followed by an induction, as seen with an increased ratio of TS protein over TS-mRNA. The clear retrospective relation between TS levels and response now forms the basis for a prospective study, in which TS levels are measured before treatment in order to determine the treatment protocol.
An alternative molecular mechanism of action of 5-fluorouracil, a potent anticancer drug.[Pubmed:9264308]
Biochem Pharmacol. 1997 Jun 1;53(11):1569-75.
It is assumed that the primary mode of action of 5-fluorouracil (5-FUra) is mediated via inhibition of thymidylate synthetase. Persistent inhibition of cellular proliferation after treatment of the 5-FUra-inhibited cells with exogenous thymidine do not support the notion that the anti-proliferitive action of 5-FUra is due exclusively to inhibition of DNA replication. Our studies have revealed an alternative mechanism of action at the level of pre-ribosomal RNA (pre-rRNA) processing. Pre-rRNA processing was inhibited completely in vitro as well as in S-100 extract from the mouse lymphosarcoma P1798 cells that were treated with 5-FUra. Under this condition, the 5-FUra-substituted pre-rRNA substrate was processed efficiently at the primary processing site. This study showed that the activity and/or the synthesis of a factor potentially involved in pre-rRNA processing is blocked in cells treated with the fluoropyrimidine. UV-cross-linking study showed that a 200 kDa polypeptide designated ribosomal RNA binding protein (RRBP) was absent in the S-100 extract from the drug-treated mouse lymphosarcoma cells. Since a polypeptide that cross-links to a processing site on RNA is usually involved in the RNA processing, RRBP may have a direct role in pre-rRNA processing. A key molecular mechanism far the antiproliferative action of 5-FUra may be due to its interference with the activity and/or synthesis of RRBP. Exposure of cells to 5-FUra did not inhibit the interaction between U3 small nucleolar RNA (snoRNA) and pre-rRNA at the primary processing site (a key step in the processing reaction) and the formation of U3 small nucleolar ribonucleoprotein (snoRNP). Treatment of cells with the fluoropyrimidine did not block the 3' end processing of pre-messenger RNA (pre-mRNA). This article also discusses the effects of 5-FUra on pre-mRNA splicing and mRNA translation, and proposes other avenues of research to explore further the mechanism of action of this important pyrimidine analog.


