VoacamineCAS# 3371-85-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3371-85-5 | SDF | Download SDF |
| PubChem ID | 11953931 | Appearance | White crystalline powder |
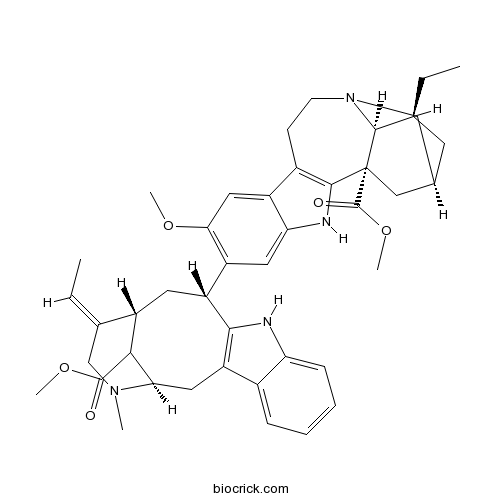
| Formula | C43H52N4O5 | M.Wt | 704.91 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl (1S,15S,17S,18S)-17-ethyl-6-[(1R,12R,14R,15E)-15-ethylidene-18-methoxycarbonyl-17-methyl-10,17-diazatetracyclo[12.3.1.03,11.04,9]octadeca-3(11),4,6,8-tetraen-12-yl]-7-methoxy-3,13-diazapentacyclo[13.3.1.02,10.04,9.013,18]nonadeca-2(10),4,6,8-tetraene-1-carboxylate | ||
| SMILES | CCC1CC2CC3(C1N(C2)CCC4=C3NC5=CC(=C(C=C45)OC)C6CC7C(C(CC8=C6NC9=CC=CC=C89)N(CC7=CC)C)C(=O)OC)C(=O)OC | ||
| Standard InChIKey | VCMIRXRRQJNZJT-XRMSBCOFSA-N | ||
| Standard InChI | InChI=1S/C43H52N4O5/c1-7-24-15-23-20-43(42(49)52-6)39-27(13-14-47(21-23)40(24)43)29-19-36(50-4)30(17-34(29)45-39)31-16-28-25(8-2)22-46(3)35(37(28)41(48)51-5)18-32-26-11-9-10-12-33(26)44-38(31)32/h8-12,17,19,23-24,28,31,35,37,40,44-45H,7,13-16,18,20-22H2,1-6H3/b25-8-/t23-,24-,28-,31+,35+,37?,40-,43+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Voacanginine may have rhythmic effects. |
| In vivo | A propos des propriétés cardiotoniques de la voacangine et de la voacanginine.[Pubmed: 13260772]Bull Acad R Med Belg. 1955;20(5):194-216.A propos des propriétés cardiotoniques de la voacangine et de la Voacanginine. |

Voacamine Dilution Calculator

Voacamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4186 mL | 7.0931 mL | 14.1862 mL | 28.3724 mL | 35.4655 mL |
| 5 mM | 0.2837 mL | 1.4186 mL | 2.8372 mL | 5.6745 mL | 7.0931 mL |
| 10 mM | 0.1419 mL | 0.7093 mL | 1.4186 mL | 2.8372 mL | 3.5466 mL |
| 50 mM | 0.0284 mL | 0.1419 mL | 0.2837 mL | 0.5674 mL | 0.7093 mL |
| 100 mM | 0.0142 mL | 0.0709 mL | 0.1419 mL | 0.2837 mL | 0.3547 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (-)-Gallocatechin
Catalog No.:BCN5927
CAS No.:3371-27-5
- Pachypodol
Catalog No.:BCN5261
CAS No.:33708-72-4
- 19,20-(E)-Vallesamine
Catalog No.:BCN5260
CAS No.:3368-87-4
- Hypophyllanthin
Catalog No.:BCN5259
CAS No.:33676-00-5
- Secnidazole
Catalog No.:BCC4971
CAS No.:3366-95-8
- H-Thr-Obzl.HCl
Catalog No.:BCC2674
CAS No.:33645-24-8
- (S)-(+)-Ketamine hydrochloride
Catalog No.:BCC7930
CAS No.:33643-47-9
- 1-O-Acetylbritannilactone
Catalog No.:BCN7715
CAS No.:33627-41-7
- Britannin
Catalog No.:BCN2366
CAS No.:33627-28-0
- Desoxyrhapontigenin
Catalog No.:BCN6479
CAS No.:33626-08-3
- Britannilactone
Catalog No.:BCN3509
CAS No.:33620-72-3
- Ispinesib (SB-715992)
Catalog No.:BCC2509
CAS No.:336113-53-2
- 4,4'-Di-O-methylellagic acid
Catalog No.:BCN3709
CAS No.:3374-77-4
- Cannabivarin
Catalog No.:BCN7587
CAS No.:33745-21-0
- Abieta-8,11,13-triene-7,15,18-triol
Catalog No.:BCN5262
CAS No.:337527-10-3
- Androstanolone heptanoate
Catalog No.:BCC8827
CAS No.:33776-88-4
- Hederacholchiside E
Catalog No.:BCC8329
CAS No.:33783-82-3
- Deodarin
Catalog No.:BCN6874
CAS No.:33788-39-5
- H-D-Abu-OH
Catalog No.:BCC3202
CAS No.:338-69-2
- Methasterone
Catalog No.:BCC9027
CAS No.:3381-88-2
- Di-O-methylbergenin
Catalog No.:BCN5263
CAS No.:33815-57-5
- CITCO
Catalog No.:BCC7749
CAS No.:338404-52-7
- LY 456236 hydrochloride
Catalog No.:BCC7347
CAS No.:338736-46-2
- Demethyl tetrandrine
Catalog No.:BCN2624
CAS No.:33889-68-8
Voacamine modulates the sensitivity to doxorubicin of resistant osteosarcoma and melanoma cells and does not induce toxicity in normal fibroblasts.[Pubmed:24720452]
J Nat Prod. 2014 Apr 25;77(4):855-62.
In previous studies it has been demonstrated that the plant alkaloid Voacamine (1), used at noncytotoxic concentrations, enhanced the cytotoxicity of doxorubicin and exerted a chemosensitizing effect on cultured multidrug-resistant (MDR) U-2 OS-DX osteosarcoma cells. The in vitro investigations reported herein gave the following results: (i) the chemosensitizing effect of 1, in terms of drug accumulation and cell survival, was confirmed using SAOS-2-DX cells, another MDR osteosarcoma cell line; (ii) compound 1 enhanced the cytotoxic effect of doxorubicin also on the melanoma cell line Me30966, intrinsically drug resistant and P-glycoprotein-negative; (iii) at the concentrations used to sensitize tumor cells, 1 was not cytotoxic to normal cells (human fibroblasts). These findings suggest possible applications of Voacamine (1) in integrative oncologic therapies against resistant tumors.
Voacamine alters Leishmania ultrastructure and kills parasite by poisoning unusual bi-subunit topoisomerase IB.[Pubmed:28483460]
Biochem Pharmacol. 2017 Aug 15;138:19-30.
Indole alkaloids possess a large spectrum of biological activities including anti-protozoal action. Here we report for the first time that Voacamine, isolated from the plant Tabernaemontana coronaria, is an antiprotozoal agent effective against a large array of trypanosomatid parasites including Indian strain of Leishmania donovani and Brazilian strains of Leishmania amazonensis and Trypanosoma cruzi. It inhibits the relaxation activity of topoisomerase IB of L. donovani (LdTop1B) and stabilizes the cleavable complex. Voacamine is probably the first LdTop1B-specific poison to act uncompetitively. It has no impact on human topoisomerase I and II up to 200muM concentrations. The study also provides a thorough insight into ultrastructural alterations induced in three kinetoplastid parasites by a specific inhibitor of LdTop1B. Voacamine is also effective against intracellular amastigotes of different drug unresponsive field isolates of Leishmania donovani obtained from endemic zones of India severely affected with visceral leishmaniasis. Most importantly, this is the first report demonstrating the efficacy of a compound to reduce the burden of drug resistant parasites, unresponsive to SAG, amphotericin B and miltefosine, in experimental BALB/c mice model of visceral leishmaniasis. The findings cumulatively provide a strong evidence that Voacamine can be a promising drug candidate against trypanosomatid infections.
High-performance thin-layer chromatography for the evaluation of voacamine intracellular concentration related to its cytotoxic effect.[Pubmed:26298394]
J Pharm Biomed Anal. 2015 Nov 10;115:467-74.
Previous investigations demonstrated that pretreatment with non-cytotoxic concentrations of Voacamine had a chemosensitizing effect on cultured multidrug resistant osteosarcoma cells exposed to doxorubicin; whereas when used alone at high concentrations Voacamine induced apoptosis-independent cell death on both sensitive and resistant cells. To gain insight into the mechanism of action of Voacamine at the subcellular level, we developed an analytical high-performance thin-layer chromatography technique to assess the intracellular content of Voacamine that could be correlated with the induction of cell death and consequent morphological and ultrastructural changes. The results of the quantitative analysis not only did allow us to measure both the amount of unmodified Voacamine molecules (determined by the method) and the amount of molecules which reacted with cellular components (undetectable), but also to confirm the findings of our previous studies and support the validity of this method.
Phytochemical investigations and evaluation of antimutagenic activity of the alcoholic extract of Glycosmis pentaphylla and Tabernaemontana coronaria by Ames test.[Pubmed:28423921]
Nat Prod Res. 2018 Mar;32(5):582-587.
Chemical investigation of root bark of Glycosmis pentaphylla and stem bark of Tabernaemontana coronaria led to the isolation of three carbazole alkaloids glycozoline, glycozolidine and methyl carbazole 3-carboxylate, two furoquinoline alkaloids skimmianine and dictamine, an acridone alkaloid arborinine, three monomeric indole alkaloids coronaridine, 10-methoxy coronaridine and tabernaemontanine, and two dimeric indole alkaloids Voacamine and tabernaelegantine B. Their structures were established by detailed spectral analysis. Mutagenic and antimutagenic potential of methanol extract of both plant materials were evaluated by Ames test against known positive mutagens 2-aminofluorine, 4-nitro-O-phenylenediamine and sodium azide using Salmonella typhimurium TA 98 and TA 100 bacterial strains both in the presence and absence of S9. Both the extracts were non-mutagenic in nature. Both the extracts of G. pentaphylla and T. coronaria exhibited significant antimutagenic activity against NPD and sodium azide for S. typhimurium TA98 and TA100 strains. The results indicated that the extracts could counteract the mutagenicity induced by different genotoxic compounds.
Cytotoxic monoterpenoid indole alkaloids isolated from the barks of Voacanga africana Staph.[Pubmed:26140390]
Nat Prod Res. 2016;30(10):1144-9.
A new monoterpenoid indole alkaloid compound (1) and six known monoterpenoid indole alkaloids compounds (2-7) were isolated from the barks of Voacanga africana Staph. The structures were established by spectral analysis as ibogamine-16-carboxylic acid,17,20-didehydro-5,6-dioxo-10-methoxy-methyl ester (1), Voacamine (2), vobasine (3), voacangine (4), voacristine (5), 19-epi-voacristine (6) and 19-epi-heyneanine (7). Compound 1 was confirmed by X-ray crystallographic analysis. All of the isolated compounds were evaluated for cytotoxicity against five cell lines (HEPG-2, A375, MDA-MB-231, SH-SY5Y, CT26). Among them, compounds 2 and 6 displayed significant inhibitory activities, compounds 3, 4 and 5 showed moderate inhibitory activities, while compounds 1 and 7 showed no inhibitory activities against the five cell lines.
Discovery of indole alkaloids with cannabinoid CB1 receptor antagonistic activity.[Pubmed:21376588]
Bioorg Med Chem Lett. 2011 Apr 1;21(7):1962-4.
Three indole alkaloids, Voacamine (1), 3,6-oxidovoacangine (2), and a new alkaloid, 5-hydroxy-3,6-oxidovoacangine (3), isolated from Voacanga africana were found to exhibit potent cannabinoid CB1 receptor antagonistic activity. This is the first example of CB1 antagonists derived from natural alkaloids.


