BiotinLysine probe coupled to mass spectrometry detection CAS# 58-85-5 |
- Sulfo-NHS-Biotin
Catalog No.:BCC3576
CAS No.:119616-38-5
- Sulfo-NHS-LC-Biotin
Catalog No.:BCC3578
CAS No.:127062-22-0
- Biotin-HPDP
Catalog No.:BCC3583
CAS No.:129179-83-5
- NHS-SS-Biotin
Catalog No.:BCC3581
CAS No.:142439-92-7
- Sulfo-NHS-SS-Biotin
Catalog No.:BCC3580
CAS No.:325143-98-4
- NHS-LC-Biotin
Catalog No.:BCC3579
CAS No.:72040-63-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 58-85-5 | SDF | Download SDF |
| PubChem ID | 253 | Appearance | Powder |
| Formula | C10H16N2O3S | M.Wt | 244.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | D-Biotin,Vitamin H | ||
| Solubility | DMSO : 41.67 mg/mL (170.56 mM; Need ultrasonic) | ||
| Chemical Name | 5-(2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl)pentanoic acid | ||
| SMILES | C1C2C(C(S1)CCCCC(=O)O)NC(=O)N2 | ||
| Standard InChIKey | YBJHBAHKTGYVGT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H16N2O3S/c13-8(14)4-2-1-3-7-9-6(5-16-7)11-10(15)12-9/h6-7,9H,1-5H2,(H,13,14)(H2,11,12,15) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
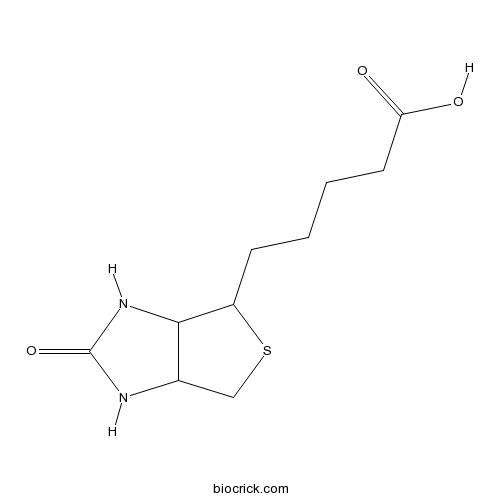
| Description | Biotin is a water-soluble, enzyme co-factor present in minute amounts in every living cell.
Target: Others
Biotin is necessary for cell growth, the production of fatty acids, and the metabolism of fats and amino acids. It plays a role in the citric acid cycle, which is the process by which biochemical energy is generated during aerobic respiration. Biotin is a coenzyme for carboxylase enzymes, involved in the synthesis of fatty acids, isoleucine, and valine, and in gluconeogenesis. In addition, biotin is widely used throughout the biotechnology industry to conjugate proteins for biochemical assays. The dietary biotin intake in Western populations has been estimated to be 35 to 70 microg/d (143-287 nmol/d). Recent studies suggest that humans absorb biotin nearly completely. Conditions that may increase biotin requirements in humans include pregnancy, lactation, and therapy with anticonvulsants or lipoic acid [1, 2]. References: | |||||

Biotin Dilution Calculator

Biotin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0932 mL | 20.4658 mL | 40.9316 mL | 81.8632 mL | 102.329 mL |
| 5 mM | 0.8186 mL | 4.0932 mL | 8.1863 mL | 16.3726 mL | 20.4658 mL |
| 10 mM | 0.4093 mL | 2.0466 mL | 4.0932 mL | 8.1863 mL | 10.2329 mL |
| 50 mM | 0.0819 mL | 0.4093 mL | 0.8186 mL | 1.6373 mL | 2.0466 mL |
| 100 mM | 0.0409 mL | 0.2047 mL | 0.4093 mL | 0.8186 mL | 1.0233 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
NHS-biotin modification as a specific lysine probe coupled to mass spectrometry detection is increasingly used over the past years for assessing amino acid accessibility of proteins or complexes as an alternative when well-established methods are challenged1.
Numerous applications have already been reported2, with increasing use of N-HydroxySuccinimide- biotin (NHS-biotin) as a specific lysine probe3. As an example, determination of surface accessibility of amino acids may be useful to delineate protein–protein interfaces as shown in epitopemapping4.
Labeling of amino acids containing hydroxyl groups has been already observed for the Sulfo-NHS-biotin reagent reacting on model peptides33 and for 3,30-Dithiobis[sulfosuccinimidyl propionate] (DTSSP)5.It has been shown that serine, tyrosine, and threonine residues readily react with Sulfo- NHS-biotin if the hydroxyl-containing group is located two positions from a histidine residue6. It was proposed that the enhanced intrinsic reactivity of these hydroxyl groups was due to an interaction with the histidyl residue via hydrogen bonding that contributes to increase their nucleophilicity.
The pioneering observationsmade with DTSSP are also detected with NHS-biotin derivatives: hydroxyl groups of serine and tyrosine residues also reactwith primary amine reagents and serine label may be lost resulting in a dehydrated peptide. Use of biotin reagents allows affinity enrichment by means of streptavidin3, as well as absolute quantitation of the labels introduced per polypeptide by means of a spectrophotometric assay.
Use of the complete set of Sulfo-NHS-biotin reagents allows an efficient and reliable assignment of the different ions detected.
Reference:
1. G. Gabant, J. Augier et al, Assessment of solvent residues accessibility using three Sulfo-NHS-biotin reagents in parallel: application to footprint changes of a methyltransferase upon binding its substrate, J. Mass Spectrom. 2008; 43: 360–370
2. Glocker MO, Borchers C, Fiedler W, Suckau D, Przybylski M. Molecular characterization of surface topology in protein tertiary structures by amino-acylation and mass spectrometric peptide mapping. Bioconjugate Chem. 1994; 5: 583.
3. Azim-Zadeh O, Hillebrecht A, Linne U, Marahiel MA, Klebe G, Lingelbach K, Nyalwidhe J. Use of biotin derivatives to probe conformational changes in proteins. Journal of Biological Chemistry 2007; 282: 21609
4. Borch J, Jorgensen TJ, Roepstorff P. Mass spectrometric analysis of protein interactions. Current Opinion in Chemical Biology2005; 9: 509.
5. Swaim CL, Smith JB, Smith DL. Unexpected products from the reaction of the synthetic cross-linker 3, 3’ dithiobis(sulfosuccinimidyl propionate), DTSSP with peptides. Journal of the American Society for Mass Spectrometry 2004; 15:736.
6. Miller BT, Kurosky A. Elevated intrinsic reactivity of seryl hydroxyl groups within the linear peptide triads His-Xaa-Ser or Ser-Xaa-His. Biochemical and Biophysical Research Communications 1993; 196: 461.
- Papaverine
Catalog No.:BCC8230
CAS No.:58-74-2
- Inosine
Catalog No.:BCN3841
CAS No.:58-63-9
- Adenosine
Catalog No.:BCN5796
CAS No.:58-61-7
- Puromycin aminonucleoside
Catalog No.:BCC1873
CAS No.:58-60-6
- Puromycin dihydrochloride
Catalog No.:BCC7860
CAS No.:58-58-2
- Pyridoxine HCl
Catalog No.:BCC4835
CAS No.:58-56-0
- Theophylline
Catalog No.:BCN1258
CAS No.:58-55-9
- Tetrabenazine
Catalog No.:BCC5277
CAS No.:58-46-8
- Prochlorperazine
Catalog No.:BCC3846
CAS No.:58-38-8
- Promethazine HCl
Catalog No.:BCC5480
CAS No.:58-33-3
- Desipramine hydrochloride
Catalog No.:BCC7553
CAS No.:58-28-6
- Menadione
Catalog No.:BCN8351
CAS No.:58-27-5
- D-(+)-Xylose
Catalog No.:BCN1010
CAS No.:58-86-6
- Hydrochlorothiazide
Catalog No.:BCC4786
CAS No.:58-93-5
- Chlorothiazide
Catalog No.:BCC3752
CAS No.:58-94-6
- alpha-Tocopherol acetate
Catalog No.:BCN5803
CAS No.:58-95-7
- Uridine
Catalog No.:BCN4090
CAS No.:58-96-8
- 6-Aminoquinoline
Catalog No.:BCC8766
CAS No.:580-15-4
- 3-Aminoquinoline
Catalog No.:BCC8620
CAS No.:580-17-6
- 2-Aminoquinoline
Catalog No.:BCC8555
CAS No.:580-22-3
- Matairesinol
Catalog No.:BCN5789
CAS No.:580-72-3
- Epicorynoxidine
Catalog No.:BCN7554
CAS No.:58000-48-9
- HOKU-81
Catalog No.:BCC1634
CAS No.:58020-43-2
- Averantin
Catalog No.:BCN7027
CAS No.:5803-62-3
Conjugation of biotin-coated luminescent quantum dots with single domain antibody-rhizavidin fusions.[Pubmed:28352525]
Biotechnol Rep (Amst). 2016 Mar 3;10:56-65.
Straightforward and effective methods are required for the bioconjugation of proteins to surfaces and particles. Previously we demonstrated that the fusion of a single domain antibody with the Biotin binding molecule rhizavidin provided a facile method to coat Biotin-modified surfaces with a highly active and oriented antibody. Here, we constructed similar single domain antibody-rhizavidin fusions as well as unfused rhizavidin with a His-tag. The unfused rhizavidin produced efficiently and its utility for assay development was demonstrated in surface plasmon resonance experiments. The single domain antibody-rhizavidin fusions were utilized to coat quantum dots that had been prepared with surface Biotins. Preparation of antibody coated quantum dots by this means was found to be both easy and effective. The prepared single domain antibody-quantum dot reagent was characterized by surface plasmon resonance and applied to toxin detection in a fluoroimmunoassay sensing format.
Co-expression of BirA with biotin bait achieves in vivo biotinylation of overexpressed stable N-glycosylated sRAGE in transgenic silkworms.[Pubmed:28336960]
Sci Rep. 2017 Mar 23;7(1):356.
Here, we demonstrated the expression of the N-glycosylated extracellular ligand binding domain of receptor for advanced glycation end products (sRAGE) in middle silk glands (MSGs) of transgenic silkworms using the GAL4/UAS system. Over 1 mg of sRAGE was obtained from one transgenic silkworm. sRAGE purified from the silkworm exhibited good stability and maintained specific ligand-binding ability. In addition, N-glycan analysis of sRAGE revealed that N-glucan completely lacked potentially allergenic fucose. Moreover, co-expression of Biotin ligase (BirA) with C-terminal BioEase-tagged sRAGE in MSGs resulted in efficient Biotinylation of sRAGE after addition of Biotin bait. C-terminal Biotinylated sRAGE could be immobilized onto a solid surface in one direction through binding to streptavidin without any loss of ability. The dissociation constant of sRAGE with fructose-BSA, a typical RAGE ligand, was 7.25 x 10(-7) M, consistent with that on the mammalian cell surface. Thus, we developed a novel, innovative silkworm expression system for efficient expression of recombinant sRAGE, which could serve as a basis for the elucidation of RAGE-ligand interactions and facilitate the search for new ligands and inhibitors.
Supplementation with Phycocyanobilin, Citrulline, Taurine, and Supranutritional Doses of Folic Acid and Biotin-Potential for Preventing or Slowing the Progression of Diabetic Complications.[Pubmed:28335416]
Healthcare (Basel). 2017 Mar 14;5(1). pii: healthcare5010015.
Oxidative stress, the resulting uncoupling of endothelial nitric oxide synthase (eNOS), and loss of nitric oxide (NO) bioactivity, are key mediators of the vascular and microvascular complications of diabetes. Much of this oxidative stress arises from up-regulated nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity. Phycocyanobilin (PhyCB), the light-harvesting chromophore in edible cyanobacteria such as spirulina, is a biliverdin derivative that shares the ability of free bilirubin to inhibit certain isoforms of NADPH oxidase. Epidemiological studies reveal that diabetics with relatively elevated serum bilirubin are less likely to develop coronary disease or microvascular complications; this may reflect the ability of bilirubin to ward off these complications via inhibition of NADPH oxidase. Oral PhyCB may likewise have potential in this regard, and has been shown to protect diabetic mice from glomerulosclerosis. With respect to oxidant-mediated uncoupling of eNOS, high-dose folate can help to reverse this by modulating the oxidation status of the eNOS cofactor tetrahydrobiopterin (BH4). Oxidation of BH4 yields dihydrobiopterin (BH2), which competes with BH4 for binding to eNOS and promotes its uncoupling. The reduced intracellular metabolites of folate have versatile oxidant-scavenging activity that can prevent oxidation of BH4; concurrently, these metabolites promote induction of dihydrofolate reductase, which functions to reconvert BH2 to BH4, and hence alleviate the uncoupling of eNOS. The arginine metabolite asymmetric dimethylarginine (ADMA), typically elevated in diabetics, also uncouples eNOS by competitively inhibiting binding of arginine to eNOS; this effect is exacerbated by the increased expression of arginase that accompanies diabetes. These effects can be countered via supplementation with citrulline, which efficiently enhances tissue levels of arginine. With respect to the loss of NO bioactivity that contributes to diabetic complications, high dose Biotin has the potential to "pinch hit" for diminished NO by direct activation of soluble guanylate cyclase (sGC). High-dose Biotin also may aid glycemic control via modulatory effects on enzyme induction in hepatocytes and pancreatic beta cells. Taurine, which suppresses diabetic complications in rodents, has the potential to reverse the inactivating impact of oxidative stress on sGC by boosting synthesis of hydrogen sulfide. Hence, it is proposed that concurrent administration of PhyCB, citrulline, taurine, and supranutritional doses of folate and Biotin may have considerable potential for prevention and control of diabetic complications. Such a regimen could also be complemented with antioxidants such as lipoic acid, N-acetylcysteine, and melatonin-that boost cellular expression of antioxidant enzymes and glutathione-as well as astaxanthin, zinc, and glycine. The development of appropriate functional foods might make it feasible for patients to use complex nutraceutical regimens of the sort suggested here.


