ProchlorperazineDopamine (D2) receptor antagonist CAS# 58-38-8 |
- Ibutilide Fumarate
Catalog No.:BCC5076
CAS No.:122647-32-9
- Amiloride HCl dihydrate
Catalog No.:BCC5068
CAS No.:17440-83-4
- Triamterene
Catalog No.:BCC5074
CAS No.:396-01-0
- Procaine HCl
Catalog No.:BCC5072
CAS No.:51-05-8
- Proparacaine HCl
Catalog No.:BCC5073
CAS No.:5875-06-9
- Phenytoin sodium
Catalog No.:BCC5071
CAS No.:630-93-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 58-38-8 | SDF | Download SDF |
| PubChem ID | 4917 | Appearance | Powder |
| Formula | C20H24ClN3S | M.Wt | 373.94 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
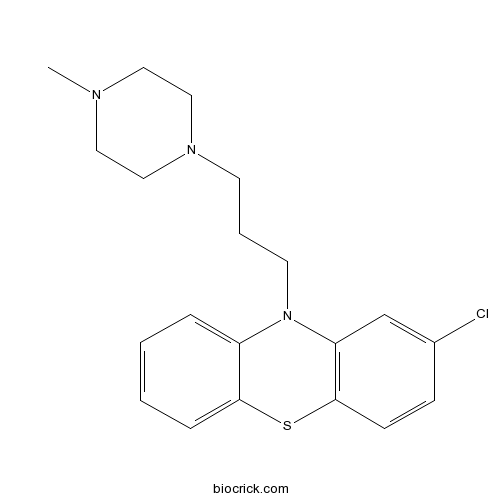
| Chemical Name | 2-chloro-10-[3-(4-methylpiperazin-1-yl)propyl]phenothiazine | ||
| SMILES | CN1CCN(CC1)CCCN2C3=CC=CC=C3SC4=C2C=C(C=C4)Cl | ||
| Standard InChIKey | WIKYUJGCLQQFNW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H24ClN3S/c1-22-11-13-23(14-12-22)9-4-10-24-17-5-2-3-6-19(17)25-20-8-7-16(21)15-18(20)24/h2-3,5-8,15H,4,9-14H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Prochlorperazine Dilution Calculator

Prochlorperazine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6742 mL | 13.3711 mL | 26.7423 mL | 53.4845 mL | 66.8556 mL |
| 5 mM | 0.5348 mL | 2.6742 mL | 5.3485 mL | 10.6969 mL | 13.3711 mL |
| 10 mM | 0.2674 mL | 1.3371 mL | 2.6742 mL | 5.3485 mL | 6.6856 mL |
| 50 mM | 0.0535 mL | 0.2674 mL | 0.5348 mL | 1.0697 mL | 1.3371 mL |
| 100 mM | 0.0267 mL | 0.1337 mL | 0.2674 mL | 0.5348 mL | 0.6686 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Prochlorperazine (Compazine, Stemzine, Buccastem, Stemetil, Phenotil) is a dopamine (D2) receptor antagonist that belongs to the phenothiazine class of antipsychotic agents that are used for the antiemetic treatment of nausea and vertigo. It is also a highly potent typical antipsychotic, 10-20x more potent than chlorpromazine. It is also used to treat migraine headaches. Intravenous administration can be used to treat status migrainosus.
- Promethazine HCl
Catalog No.:BCC5480
CAS No.:58-33-3
- Desipramine hydrochloride
Catalog No.:BCC7553
CAS No.:58-28-6
- Menadione
Catalog No.:BCN8351
CAS No.:58-27-5
- Testosterone
Catalog No.:BCN2193
CAS No.:58-22-0
- Testosterone cypionate
Catalog No.:BCC9167
CAS No.:58-20-8
- Methyltestosterone
Catalog No.:BCC9045
CAS No.:58-18-4
- Aminophenazone
Catalog No.:BCC8815
CAS No.:58-15-1
- Pyrimethamine
Catalog No.:BCC2307
CAS No.:58-14-0
- Caffeine
Catalog No.:BCN5807
CAS No.:58-08-2
- Bax inhibitor peptide P5
Catalog No.:BCC2393
CAS No.:579492-83-4
- Bax inhibitor peptide V5
Catalog No.:BCC2394
CAS No.:579492-81-2
- Officinalisinin I
Catalog No.:BCN2825
CAS No.:57944-18-0
- Tetrabenazine
Catalog No.:BCC5277
CAS No.:58-46-8
- Theophylline
Catalog No.:BCN1258
CAS No.:58-55-9
- Pyridoxine HCl
Catalog No.:BCC4835
CAS No.:58-56-0
- Puromycin dihydrochloride
Catalog No.:BCC7860
CAS No.:58-58-2
- Puromycin aminonucleoside
Catalog No.:BCC1873
CAS No.:58-60-6
- Adenosine
Catalog No.:BCN5796
CAS No.:58-61-7
- Inosine
Catalog No.:BCN3841
CAS No.:58-63-9
- Papaverine
Catalog No.:BCC8230
CAS No.:58-74-2
- Biotin
Catalog No.:BCC3585
CAS No.:58-85-5
- D-(+)-Xylose
Catalog No.:BCN1010
CAS No.:58-86-6
- Hydrochlorothiazide
Catalog No.:BCC4786
CAS No.:58-93-5
- Chlorothiazide
Catalog No.:BCC3752
CAS No.:58-94-6
Prochlorperazine-Induced Hemidystonia Mimicking Acute Stroke.[Pubmed:26265971]
West J Emerg Med. 2015 Jul;16(4):572-4.
Prochlorperazine is frequently used in the treatment of refractory nausea and migraines. Known side effects include extrapyramidal symptoms such as akathisia and dystonia. We report a pregnant patient taking Prochlorperazine for hyperemesis gravidarum who developed hemidystonia, which triggered an acute code stroke response from prehospital, emergency medicine and neurology providers. We suspect this report to be the first case of Prochlorperazine-induced hemidystonia as a stroke mimic.
Incidence of nausea and vomiting induced by oxycodone administered with prochlorperazine in Japanese cancer patients.[Pubmed:25959201]
J Nippon Med Sch. 2015;82(2):100-5.
BACKGROUND: Nausea and vomiting are the most frequent side effects of opioids and may cause the opioids to be discontinued. New methods for preventing opioid-induced nausea can improve cancer pain management. Oxycodone is one of the most frequently used opioid used in Japan because patients receiving oxycodone report less nausea and vomiting than do patients receiving morphine. The reported incidence of oxycodone-induced nausea varies widely, although the true incidence remains unclear. As a first step toward preventing oxycodone-induced nausea, we aimed to determine the incidence of and risk factors for oxycodone-induced nausea and vomiting. METHODS: In this observational study, we analyzed a series of consecutive inpatients with cancer who received oxycodone with Prochlorperazine as a preventive antiemetic agent. Oxycodone (5 mg) was administered either at 08:00 and 20:00 or at 09:00 and 21:00, and Prochlorperazine (5 mg) was also given at the same times for 5 days. RESULTS: Of the 145 enrolled patients, 138 were suitable for analysis. The incidence of nausea was 18.1%, and that of vomiting was 5.8%. The incidence of nausea was higher, but not to a significant degree, in women than in men (P=0.07). Furthermore, the incidence of vomiting in women was equal to that in men (P=0.28), whereas the incidences of both nausea (P=0.99) and vomiting (P=0.89) in elderly patients were equal to those in younger patients. In addition, the incidence of nausea (P=0.52) and vomiting (P=0.91) in patients with digestive system cancer was equal to that of patients with non-digestive system cancer. CONCLUSIONS: The incidence of nausea induced by oxycodone with Prochlorperazine was 18.1% in opioid-naive Japanese inpatients. Female sex may be a risk factor for oxycodone-induced nausea. These results suggest that a clinical study would require 314 participants (157 in each group) to decrease the incidence from 18% to 8% (10% decrease) with a new preventive treatment (alpha error=0.05, beta error=0.2).
The Safety of Prochlorperazine in Children: A Systematic Review and Meta-Analysis.[Pubmed:26884326]
Drug Saf. 2016 Jun;39(6):509-16.
INTRODUCTION: Prochlorperazine is recommended for adults with breakthrough or refractory chemotherapy-induced nausea and vomiting (CINV). The objective of this review was to describe its safety in children when given for any indication to help define its role for CINV control in children. METHODS: Electronic searches of MEDLINE, EMBASE, PsycINFO, and the Cochrane Central Register of Controlled Trials were performed as of 9 March 2015. All studies in English reporting adverse effects (AEs) associated with Prochlorperazine in children (Prochlorperazine in 758 children were included. The most commonly reported AEs in prospective studies of Prochlorperazine in children were sedation (multiple-dose studies: 10 %, 95 % CI 5-21) and extrapyramidal symptoms (EPS) (single-dose studies: 9 %, 95 % CI 3-29; multiple-dose studies: 4 %, 95 % CI 1-11). Serious AEs (seizure, neuroleptic malignant syndrome, autonomic collapse, tardive dyskinesia) were rarely associated with Prochlorperazine use in children. Five fatalities were reported in children receiving Prochlorperazine. LIMITATIONS: The limitations of this systematic review and meta-analysis were that the AEs reported in the included studies were heterogeneous, the prospective use of systematic clinical tools to identify AEs was rare, and the risk of bias in most prospective studies was moderate. CONCLUSIONS: The most common AEs reported with the pediatric use of Prochlorperazine are EPS and sedation. Fatalities, life-threatening, and persistent AEs have also been reported.


