2-AminoquinolineCAS# 580-22-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 580-22-3 | SDF | Download SDF |
| PubChem ID | 11379 | Appearance | Powder |
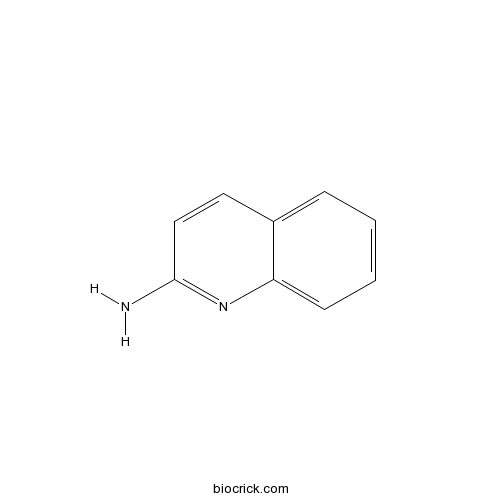
| Formula | C9H8N2 | M.Wt | 144 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | quinolin-2-amine | ||
| SMILES | C1=CC=C2C(=C1)C=CC(=N2)N | ||
| Standard InChIKey | GCMNJUJAKQGROZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H8N2/c10-9-6-5-7-3-1-2-4-8(7)11-9/h1-6H,(H2,10,11) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2-Aminoquinoline Dilution Calculator

2-Aminoquinoline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.9444 mL | 34.7222 mL | 69.4444 mL | 138.8889 mL | 173.6111 mL |
| 5 mM | 1.3889 mL | 6.9444 mL | 13.8889 mL | 27.7778 mL | 34.7222 mL |
| 10 mM | 0.6944 mL | 3.4722 mL | 6.9444 mL | 13.8889 mL | 17.3611 mL |
| 50 mM | 0.1389 mL | 0.6944 mL | 1.3889 mL | 2.7778 mL | 3.4722 mL |
| 100 mM | 0.0694 mL | 0.3472 mL | 0.6944 mL | 1.3889 mL | 1.7361 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-Aminoquinoline
Catalog No.:BCC8620
CAS No.:580-17-6
- 6-Aminoquinoline
Catalog No.:BCC8766
CAS No.:580-15-4
- Uridine
Catalog No.:BCN4090
CAS No.:58-96-8
- alpha-Tocopherol acetate
Catalog No.:BCN5803
CAS No.:58-95-7
- Chlorothiazide
Catalog No.:BCC3752
CAS No.:58-94-6
- Hydrochlorothiazide
Catalog No.:BCC4786
CAS No.:58-93-5
- D-(+)-Xylose
Catalog No.:BCN1010
CAS No.:58-86-6
- Biotin
Catalog No.:BCC3585
CAS No.:58-85-5
- Papaverine
Catalog No.:BCC8230
CAS No.:58-74-2
- Inosine
Catalog No.:BCN3841
CAS No.:58-63-9
- Adenosine
Catalog No.:BCN5796
CAS No.:58-61-7
- Puromycin aminonucleoside
Catalog No.:BCC1873
CAS No.:58-60-6
- Matairesinol
Catalog No.:BCN5789
CAS No.:580-72-3
- Epicorynoxidine
Catalog No.:BCN7554
CAS No.:58000-48-9
- HOKU-81
Catalog No.:BCC1634
CAS No.:58020-43-2
- Averantin
Catalog No.:BCN7027
CAS No.:5803-62-3
- 24, 25-Dihydroxy VD2
Catalog No.:BCC1302
CAS No.:58050-55-8
- Miltefosine
Catalog No.:BCC4360
CAS No.:58066-85-6
- trans-3,4-Methylenedioxycinnamyl alcohol
Catalog No.:BCN1410
CAS No.:58095-76-4
- α-MSH
Catalog No.:BCC7420
CAS No.:581-05-5
- Suberosin
Catalog No.:BCN5791
CAS No.:581-31-7
- Anatabine
Catalog No.:BCN6899
CAS No.:581-49-7
- Isonicoteine
Catalog No.:BCN2152
CAS No.:581-50-0
- Undulatoside A
Catalog No.:BCN6773
CAS No.:58108-99-9
Hydrophilic, Potent, and Selective 7-Substituted 2-Aminoquinolines as Improved Human Neuronal Nitric Oxide Synthase Inhibitors.[Pubmed:28776992]
J Med Chem. 2017 Aug 24;60(16):7146-7165.
Neuronal nitric oxide synthase (nNOS) is a target for development of antineurodegenerative agents. Most nNOS inhibitors mimic l-arginine and have poor bioavailability. 2-Aminoquinolines showed promise as bioavailable nNOS inhibitors but suffered from low human nNOS inhibition, low selectivity versus human eNOS, and significant binding to other CNS targets. We aimed to improve human nNOS potency and selectivity and reduce off-target binding by (a) truncating the original scaffold or (b) introducing a hydrophilic group to interrupt the lipophilic, promiscuous pharmacophore and promote interaction with human nNOS-specific His342. We synthesized both truncated and polar 2-Aminoquinoline derivatives and assayed them against recombinant NOS enzymes. Although aniline and pyridine derivatives interact with His342, benzonitriles conferred the best rat and human nNOS inhibition. Both introduction of a hydrophobic substituent next to the cyano group and aminoquinoline methylation considerably improved isoform selectivity. Most importantly, these modifications preserved Caco-2 permeability and reduced off-target CNS binding.
Nitrile in the Hole: Discovery of a Small Auxiliary Pocket in Neuronal Nitric Oxide Synthase Leading to the Development of Potent and Selective 2-Aminoquinoline Inhibitors.[Pubmed:28422508]
J Med Chem. 2017 May 11;60(9):3958-3978.
Neuronal nitric oxide synthase (nNOS) inhibition is a promising strategy to treat neurodegenerative disorders, but the development of nNOS inhibitors is often hindered by poor pharmacokinetics. We previously developed a class of membrane-permeable 2-Aminoquinoline inhibitors and later rearranged the scaffold to decrease off-target binding. However, the resulting compounds had decreased permeability, low human nNOS activity, and low selectivity versus human eNOS. In this study, 5-substituted phenyl ether-linked aminoquinolines and derivatives were synthesized and assayed against purified NOS isoforms. 5-Cyano compounds are especially potent and selective rat and human nNOS inhibitors. Activity and selectivity are mediated by the binding of the cyano group to a new auxiliary pocket in nNOS. Potency was enhanced by methylation of the quinoline and by introduction of simple chiral moieties, resulting in a combination of hydrophobic and auxiliary pocket effects that yielded high ( approximately 500-fold) n/e selectivity. Importantly, the Caco-2 assay also revealed improved membrane permeability over previous compounds.
Phenyl Ether- and Aniline-Containing 2-Aminoquinolines as Potent and Selective Inhibitors of Neuronal Nitric Oxide Synthase.[Pubmed:26469213]
J Med Chem. 2015 Nov 12;58(21):8694-712.
Excess nitric oxide (NO) produced by neuronal nitric oxide synthase (nNOS) is implicated in neurodegenerative disorders. As a result, inhibition of nNOS and reduction of NO levels is desirable therapeutically, but many nNOS inhibitors are poorly bioavailable. Promising members of our previously reported 2-Aminoquinoline class of nNOS inhibitors, although orally bioavailable and brain-penetrant, suffer from unfavorable off-target binding to other CNS receptors, and they resemble known promiscuous binders. Rearranged phenyl ether- and aniline-linked 2-Aminoquinoline derivatives were therefore designed to (a) disrupt the promiscuous binding pharmacophore and diminish off-target interactions and (b) preserve potency, isoform selectivity, and cell permeability. A series of these compounds was synthesized and tested against purified nNOS, endothelial NOS (eNOS), and inducible NOS (iNOS) enzymes. One compound, 20, displayed high potency, selectivity, and good human nNOS inhibition, and retained some permeability in a Caco-2 assay. Most promisingly, CNS receptor counterscreening revealed that this rearranged scaffold significantly reduces off-target binding.
Simplified 2-aminoquinoline-based scaffold for potent and selective neuronal nitric oxide synthase inhibition.[Pubmed:24472039]
J Med Chem. 2014 Feb 27;57(4):1513-30.
Since high levels of nitric oxide (NO) are implicated in neurodegenerative disorders, inhibition of the neuronal isoform of nitric oxide synthase (nNOS) and reduction of NO levels are therapeutically desirable. Nonetheless, many nNOS inhibitors mimic l-arginine and are poorly bioavailable. 2-Aminoquinoline-based scaffolds were designed with the hope that they could (a) mimic aminopyridines as potent, isoform-selective arginine isosteres and (b) possess chemical properties more conducive to oral bioavailability and CNS penetration. A series of these compounds was synthesized and assayed against purified nNOS enzymes, endothelial NOS (eNOS), and inducible NOS (iNOS). Several compounds built on a 7-substituted 2-Aminoquinoline core are potent and isoform-selective; X-ray crystallography indicates that aminoquinolines exert inhibitory effects by mimicking substrate interactions with the conserved active site glutamate residue. The most potent and selective compounds, 7 and 15, were tested in a Caco-2 assay and showed good permeability and low efflux, suggesting high potential for oral bioavailability.


