BMY 45778Type I IL-1 receptor antagonist CAS# 152575-66-1 |
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- Roscovitine (Seliciclib,CYC202)
Catalog No.:BCC1105
CAS No.:186692-46-6
- Nu 6027
Catalog No.:BCC1154
CAS No.:220036-08-8
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- AT7519 Hydrochloride
Catalog No.:BCC1376
CAS No.:902135-91-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 152575-66-1 | SDF | Download SDF |
| PubChem ID | 127861 | Appearance | Powder |
| Formula | C26H18N2O5 | M.Wt | 438.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO | ||
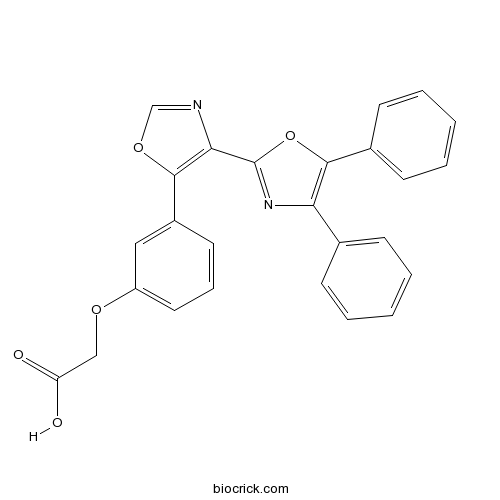
| Chemical Name | 2-[3-[4-(4,5-diphenyl-1,3-oxazol-2-yl)-1,3-oxazol-5-yl]phenoxy]acetic acid | ||
| SMILES | C1=CC=C(C=C1)C2=C(OC(=N2)C3=C(OC=N3)C4=CC(=CC=C4)OCC(=O)O)C5=CC=CC=C5 | ||
| Standard InChIKey | DSRSEEYZGWTODH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H18N2O5/c29-21(30)15-31-20-13-7-12-19(14-20)24-23(27-16-32-24)26-28-22(17-8-3-1-4-9-17)25(33-26)18-10-5-2-6-11-18/h1-14,16H,15H2,(H,29,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Non-prostanoid prostacyclin mimetic that acts as a partial agonist at IP1 prostacyclin receptors. Potently inhibits platelet aggregation in vitro (IC50 = 27-35 nM). |

BMY 45778 Dilution Calculator

BMY 45778 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2808 mL | 11.4041 mL | 22.8081 mL | 45.6163 mL | 57.0203 mL |
| 5 mM | 0.4562 mL | 2.2808 mL | 4.5616 mL | 9.1233 mL | 11.4041 mL |
| 10 mM | 0.2281 mL | 1.1404 mL | 2.2808 mL | 4.5616 mL | 5.702 mL |
| 50 mM | 0.0456 mL | 0.2281 mL | 0.4562 mL | 0.9123 mL | 1.1404 mL |
| 100 mM | 0.0228 mL | 0.114 mL | 0.2281 mL | 0.4562 mL | 0.5702 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BMY 45778 is a partial agonist of prostacyclin receptor [1].
Prostacyclin receptor (IP1) is a G-protein coupled receptor for prostacyclin. Prostacyclin inhibits platelet aggregation and elicits a potent vasodilation through binding to this receptor.
BMY 45778 is a partial agonist of prostacyclin receptor. BMY 45778 inhibited platelet aggregation with IC50 values of 35 nM, 136 nM and 1.3 μM in human, rabbit and rat, respectively. In human platelet membrane, BMY 45778 activated adenylyl cyclase with ED50 value of 6-10 nM and stimulated GTPase. Also, BMY 45778 completely inhibited the binding of Iloprost to platelet membranes with IC50 value of 7 nM. BMY 45778 inhibited iloprost-stimulated GTPase, which suggested that BMY 45778 is a partial agonist of prostacyclin receptor. In whole platelets, BMY 45778 increased cAMP levels and activated cAMP-dependent protein kinase [1]. BMY 45778 (1-10 μM) inhibited rat neutrophil aggregation induced by N-formyl-methionyl-leucylphenylalanine with IC50 value of 20 nM and inhibited the spontaneous activity of rat colon by 10-20%. Also, BMY 45778(10 μM) inhibited the inhibitory effect of cicaprost on rat colon [2].
References:
[1]. Seiler SM, Brassard CL, Federici ME, et al. [3-[4-(4,5-Diphenyl-2-oxazolyl)-5-oxazolyl]phenoxy]acetic acid (BMY 45778) is a potent non-prostanoid prostacyclin partial agonist: effects on platelet aggregation, adenylyl cyclase, cAMP levels, protein kinase, and iloprost binding. Prostaglandins, 1997, 53(1): 21-35.
[2]. Wise H, Qian YM, Jones RL. A study of prostacyclin mimetics distinguishes neuronal from neutrophil IP receptors. Eur J Pharmacol, 1995, 278(3): 265-269.
- Fmoc-N-Me-Asp(OtBu)-OH
Catalog No.:BCC3212
CAS No.:152548-66-8
- PSB 1115
Catalog No.:BCC7237
CAS No.:152529-79-8
- Nebivolol
Catalog No.:BCC4332
CAS No.:152520-56-4
- Gnetin J
Catalog No.:BCN3384
CAS No.:152511-23-4
- Leachianone G
Catalog No.:BCN3308
CAS No.:152464-78-3
- N-(5-Amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidineamine
Catalog No.:BCC9059
CAS No.:152460-10-1
- N-(2-Methyl-5-nitrophenyl)-4- (pyridin-3-yl)pyrimidin-2-amine
Catalog No.:BCC9055
CAS No.:152460-09-8
- Imatinib (STI571)
Catalog No.:BCC4979
CAS No.:152459-95-5
- Fmoc-D-Phe(4-OMe)-OH
Catalog No.:BCC2632
CAS No.:152436-04-9
- SAR405
Catalog No.:BCC4006
CAS No.:1523406-39-4
- Gnetulin
Catalog No.:BCN3401
CAS No.:152340-24-4
- (S)-4-(4-Aminobenzyl)-2(1H)-oxazolidinone
Catalog No.:BCC8400
CAS No.:152305-23-2
- Buddlejasaponin IVb
Catalog No.:BCN2842
CAS No.:152580-79-5
- Boc-Thr(Bzl)-OH
Catalog No.:BCC3451
CAS No.:15260-10-3
- H-Thr(Bzl)-OBzl.oxalate
Catalog No.:BCC3105
CAS No.:15260-11-4
- Testosterone isocaproate
Catalog No.:BCC9170
CAS No.:15262-86-9
- 2-n-Propyl-4-methyl-6-(1-methylbenzimidazole-2-yl)benzimidazole
Catalog No.:BCC8584
CAS No.:152628-02-9
- 4-Methyl-2-propyl-1H-benzimidazole-6-carboxylic acid
Catalog No.:BCC8713
CAS No.:152628-03-0
- Dihydroevocarpine
Catalog No.:BCN3691
CAS No.:15266-35-0
- Evocarpine
Catalog No.:BCN7064
CAS No.:15266-38-3
- Cimiside B
Catalog No.:BCN1679
CAS No.:152685-91-1
- Lyconnotine
Catalog No.:BCN1277
CAS No.:6900-93-2
- PF-06447475
Catalog No.:BCC5589
CAS No.:1527473-33-1
- A 366
Catalog No.:BCC5624
CAS No.:1527503-11-2
[3-[4-(4,5-Diphenyl-2-oxazolyl)-5-oxazolyl]phenoxy]acetic acid (BMY 45778) is a potent non-prostanoid prostacyclin partial agonist: effects on platelet aggregation, adenylyl cyclase, cAMP levels, protein kinase, and iloprost binding.[Pubmed:9068064]
Prostaglandins. 1997 Jan;53(1):21-35.
[3-[4-(4,5-diphenyl-2-oxazolyl)-5-oxazolyl]phenoxy]acetic acid (BMY 45778) inhibits human (IC50 = 35 nM), rabbit (136 nM) and rat (1.3 microM) platelet aggregation. This compound activates adenylyl cyclase (ED50 = 6-10 nM) and stimulates GTPase in human platelet membrane preparations. The potency (EC50) of BMY 45778 stimulating adenylyl cyclase is comparable to iloprost. However, maximal stimulation of GTPase by BMY 45778 is approximately half the iloprost-stimulated activity, and BMY 45778 limits the GTPase stimulation by iloprost suggesting that BMY 45778 is a partial agonist at the IP receptor. BMY 45778 completely prevents [3H]]Iloprost binding to platelet membranes (IC50 = 7 nM). In whole platelets, BMY 45778 causes elevation of platelet cAMP levels (cAMP content doubles at 13 nM) and activation of the cAMP-dependent protein kinase (cAMP-protein kinase ratio is twice basal at 2 nM). BMY 45778 treatment of whole platelets also desensitizes the adenylyl cyclase activation by iloprost. These results indicate that BMY 45778, which is structurally different from prostacyclin and most prostacyclin agonists, acts by stimulating prostacyclin (IP) receptors.
Non-prostanoid prostacyclin mimetics as neuronal stimulants in the rat: comparison of vagus nerve and NANC innervation of the colon.[Pubmed:10683203]
Br J Pharmacol. 2000 Feb;129(4):782-90.
The spontaneous activity of the rat isolated colon is suppressed by prostacyclin analogues such as cicaprost (IC(50)=4.0 nM). Activation of prostanoid IP(1)-receptors located on NANC inhibitory neurones is involved. However, several non-prostanoids, which show medium to high IP(1) agonist potency on platelet and vascular preparations, exhibit very weak inhibitory activity on the colon. The aim of the study was to investigate this discrepancy. Firstly, we have demonstrated the very high depolarizing potency of cicaprost on the rat isolated vagus nerve (EC(50)=0.23 nM). Iloprost, taprostene and carbacyclin were 7.9, 66, and 81 fold less potent than cicaprost, indicating the presence of IP(1) as opposed to IP(2)-receptors. Three non-prostanoid prostacyclin mimetics, BMY 45778, BMY 42393 and ONO-1301, although much less potent than cicaprost (195, 990 and 1660 fold respectively), behaved as full agonists on the vagus nerve. On re-investigating the rat colon, we found that BMY 45778 (0.1 - 3 microM), BMY 42393 (3 microM) and ONO-1301 (3 microM) behaved as specific IP(1) partial agonists, but their actions required 30 - 60 min to reach steady-state and only slowly reversed on washing. This profile contrasted sharply with the rapid and readily reversible contractions elicited by a related non-prostanoid ONO-AP-324, which is an EP(3)-receptor agonist. The full versus partial agonism of the non-prostanoid prostacyclin mimetics may be explained by the markedly different IP(1) agonist sensitivities of the two rat neuronal preparations. However, the slow kinetics of the non-prostanoids on the NANC system of the colon remain unexplained, and must be taken into account when characterizing neuronal IP-receptors.
Nonprostanoid prostacyclin mimetics. 5. Structure-activity relationships associated with [3-[4-(4,5-diphenyl-2-oxazolyl)-5- oxazolyl]phenoxy]acetic acid.[Pubmed:7504734]
J Med Chem. 1993 Nov 26;36(24):3884-903.
cis-[3-[2-(4,5-Diphenyl-2-oxazolyl)ethenyl]phenoxy]acetic acid (3) was previously identified as a nonprostanoid prostacyclin (PGI2) mimetic that potently inhibits ADP-induced aggregation of human platelets with an IC50 of 0.18 microM. As part of an effort to further explore structure-activity relationships for this class of platelet inhibitor and to provide additional insight into the nonprostanoid PGI2 mimetic pharmacophore, the effect of constraining the cis-olefin moiety of 3 into various ring systems was examined. Incorporation of the cis-olefin of 3 into either an oxazole (26) or an unsubstituted pyrazole (35) heterocycle provided compounds that are equipotent with progenitor 3. However, the oxazole 11f, which is isomeric with 26, inhibits ADP-induced human platelet aggregation in vitro with an IC50 of 0.027 microM, 6-fold more potent than 3, 26, or 35. These results suggest that the central oxazole ring of 11f is functioning as more than a simple scaffold that provides optimal stereodefinition for interaction with the PGI2 receptor. The nitrogen atom of the central heterocycle of 11f is postulated to engage in hydrogen-bond formation with a donor moiety in the PGI2 receptor protein, an interaction not available to 26 due to the markedly different topology. In support of this contention, the crystal structures of 11f and 26 contain strong intermolecular hydrogen bonds between the carboxylic acid hydrogen atom and the nitrogen atom of the central oxazole ring. Although 11f and 26 are exact isosteres and could, in principle, adopt the same molecular packing arrangement in the solid state, this is not the case, and the intermolecular hydrogen-bonding interactions in 11f and 26 are accommodated by entirely different molecular packing arrangements. Incorporation of the olefin moiety of 3 into a benzene ring provided a compound, 40, over 60-fold weaker with an IC50 of 11.1 microM. The affinities of 11f, 26, 31, 32, and 40 for the human platelet PGI2 receptor, determined by displacement of [3H]iloprost, correlated with inhibition of platelet function. The solid-state structures of 11f, 26, 31, 32, and 40 were determined and revealed that the more potent compounds 11f and 26 adopt a relatively planar overall topography. In contrast, the central phenyl ring and the phenoxy ring of the weakly active compound 40 are rotated by 53 degrees from planarity. The chemical shifts of the protons of the phenoxy rings of 3, 11f, 18, 26, 31, 32, and 40 suggest that in solution 3, 11f, 18, and 26 adopt a planar conformation while 40 does not.(ABSTRACT TRUNCATED AT 400 WORDS)


