Leachianone GCAS# 152464-78-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 152464-78-3 | SDF | Download SDF |
| PubChem ID | 5275227 | Appearance | Powder |
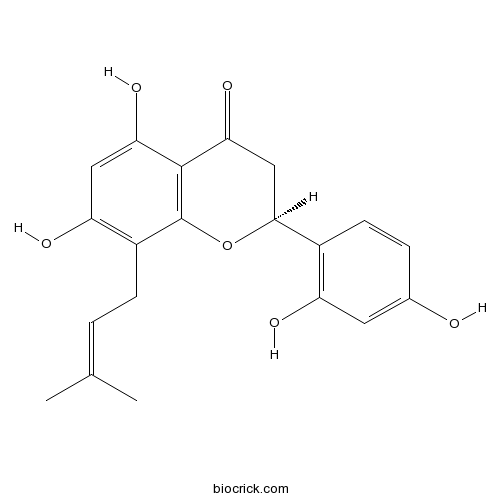
| Formula | C20H20O6 | M.Wt | 356.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-(2,4-dihydroxyphenyl)-5,7-dihydroxy-8-(3-methylbut-2-enyl)-2,3-dihydrochromen-4-one | ||
| SMILES | CC(=CCC1=C(C=C(C2=C1OC(CC2=O)C3=C(C=C(C=C3)O)O)O)O)C | ||
| Standard InChIKey | VBOYLFNGTSLAAZ-SFHVURJKSA-N | ||
| Standard InChI | InChI=1S/C20H20O6/c1-10(2)3-5-13-15(23)8-16(24)19-17(25)9-18(26-20(13)19)12-6-4-11(21)7-14(12)22/h3-4,6-8,18,21-24H,5,9H2,1-2H3/t18-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Leachianone G shows inhibitory activities against pancreatic lipase, it may be used as drug candidate to control obesity. 2. (+/-)Leachianone G 1b, bearing 8-prenyl and 2',4'-dihydoxyl groups, exhibits potent vasorelaxant and neuroprotective effects. 3. Leachianone G exhibits significant antioxidant potentials in the ABTS, ONOO(-), and total ROS assays. 4. Leachianone G shows potent antiviral activity (IC(50) = 1.6 microg/ml). |

Leachianone G Dilution Calculator

Leachianone G Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8058 mL | 14.0292 mL | 28.0584 mL | 56.1167 mL | 70.1459 mL |
| 5 mM | 0.5612 mL | 2.8058 mL | 5.6117 mL | 11.2233 mL | 14.0292 mL |
| 10 mM | 0.2806 mL | 1.4029 mL | 2.8058 mL | 5.6117 mL | 7.0146 mL |
| 50 mM | 0.0561 mL | 0.2806 mL | 0.5612 mL | 1.1223 mL | 1.4029 mL |
| 100 mM | 0.0281 mL | 0.1403 mL | 0.2806 mL | 0.5612 mL | 0.7015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N-(5-Amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidineamine
Catalog No.:BCC9059
CAS No.:152460-10-1
- N-(2-Methyl-5-nitrophenyl)-4- (pyridin-3-yl)pyrimidin-2-amine
Catalog No.:BCC9055
CAS No.:152460-09-8
- Imatinib (STI571)
Catalog No.:BCC4979
CAS No.:152459-95-5
- Fmoc-D-Phe(4-OMe)-OH
Catalog No.:BCC2632
CAS No.:152436-04-9
- SAR405
Catalog No.:BCC4006
CAS No.:1523406-39-4
- Gnetulin
Catalog No.:BCN3401
CAS No.:152340-24-4
- (S)-4-(4-Aminobenzyl)-2(1H)-oxazolidinone
Catalog No.:BCC8400
CAS No.:152305-23-2
- Amyloid β-Peptide (10-20) (human)
Catalog No.:BCC1026
CAS No.:152286-31-2
- U 93631
Catalog No.:BCC7471
CAS No.:152273-12-6
- Mupinensisone
Catalog No.:BCN4704
CAS No.:152253-67-3
- Uncargenin C
Catalog No.:BCN1678
CAS No.:152243-70-4
- GT 2016
Catalog No.:BCC7357
CAS No.:152241-24-2
- Gnetin J
Catalog No.:BCN3384
CAS No.:152511-23-4
- Nebivolol
Catalog No.:BCC4332
CAS No.:152520-56-4
- PSB 1115
Catalog No.:BCC7237
CAS No.:152529-79-8
- Fmoc-N-Me-Asp(OtBu)-OH
Catalog No.:BCC3212
CAS No.:152548-66-8
- BMY 45778
Catalog No.:BCC7068
CAS No.:152575-66-1
- Buddlejasaponin IVb
Catalog No.:BCN2842
CAS No.:152580-79-5
- Boc-Thr(Bzl)-OH
Catalog No.:BCC3451
CAS No.:15260-10-3
- H-Thr(Bzl)-OBzl.oxalate
Catalog No.:BCC3105
CAS No.:15260-11-4
- Testosterone isocaproate
Catalog No.:BCC9170
CAS No.:15262-86-9
- 2-n-Propyl-4-methyl-6-(1-methylbenzimidazole-2-yl)benzimidazole
Catalog No.:BCC8584
CAS No.:152628-02-9
- 4-Methyl-2-propyl-1H-benzimidazole-6-carboxylic acid
Catalog No.:BCC8713
CAS No.:152628-03-0
- Dihydroevocarpine
Catalog No.:BCN3691
CAS No.:15266-35-0
Re-evaluation of the antioxidant prenylated flavonoids from the roots of Sophora flavescens.[Pubmed:18451517]
Biol Pharm Bull. 2008 May;31(5):908-15.
The objective of this research was to re-evaluate the antioxidant effects of the prenylated flavonoids from Sophora flavescens via in vitro 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2'-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), peroxynitrite (ONOO(-)), and total reactive oxygen species (ROS) assays. In addition, a further examination of kuraridinol, kurarinol, and kurarinone, also isolated from S. flavescens, was carried out by the inhibition of tert-butylhydroperoxide (t-BHP)-induced intracellular ROS generation and t-BHP-induced activation of nuclear factor-kappaB (NF-kappaB). Upon re-examination of the ethyl acetate (EtOAc) soluble fraction of S. flavescens, two major prenylated chalcones, including kuraridin and kuraridinol, along with a minor prenylated flavonol, kushenol C, were isolated as good DPPH scavengers. This was in contrast to the prenylated flavanones, sophoraflavanone G and kurarinone, which were isolated from the methylene chloride (CH(2)Cl(2)) fraction of the same source. Five flavanones consisting of kushenol E, Leachianone G, kurarinol, sophoraflavanone G, and kurarinone exhibited significant antioxidant potentials in the ABTS, ONOO(-), and total ROS assays; however, the prenylated chalcones and prenylated flavonol showed more potent scavenging/inhibitory activities than the prenylated flavanones. Therefore, the prenylated chalcones and prenylated flavonol, rather than the prenylated flavanones, may make important contributions toward the marked antioxidant capacities of S. flavescens. Furthermore, kuraridinol, kurarinol, and kurarinone showed significant inhibitory activities against intracellular ROS levels as well as NF-kappaB activation by t-BHP. Overall, the results indicate that S. flavescens and its prenylated flavonoids may possess good anti-inflammatory activity, which is implicated in their significant antioxidant activity.
Synthesis, biological evaluation of prenylflavonoids as vasorelaxant and neuroprotective agents.[Pubmed:19442520]
Bioorg Med Chem Lett. 2009 Jun 15;19(12):3196-8.
A series of prenylflavonoids with multiple hydroxyl groups were synthesized and evaluated for their vasorelaxant activities against rat aorta rings pre-contracted by phenylephrine (PE), as well as their neuroprotective effects against OGD induced PC12 cell injury. The results indicated that the prenyl group at A-ring of prenylflavonoids, as well as hydroxyl groups at B-ring was important for their activities. (+/-)Leachianone G 1b, bearing 8-prenyl and 2',4'-dihydoxyl groups, exhibited the most potent vasorelaxant and neuroprotective effects.
Medicinal plant phytochemicals and their inhibitory activities against pancreatic lipase: molecular docking combined with molecular dynamics simulation approach.[Pubmed:28446025]
Nat Prod Res. 2018 May;32(10):1123-1129.
Obesity is the worst health risk worldwide, which is linked to a number of diseases. Pancreatic lipase is considered as an affective cause of obesity and can be a major target for controlling the obesity. The present study was designed to find out best phytochemicals against pancreatic lipase through molecular docking combined with molecular dynamics (MD) simulation. For this purpose, a total of 3770 phytochemicals were docked against pancreatic lipase and ranked them on the basis of binding affinity. Finally, 10 molecules (Kushenol K, Rosmarinic acid, Reserpic acid, Munjistin, Leachianone G, Cephamycin C, Arctigenin, 3-O-acetylpadmatin, Geniposide and Obtusin) were selected that showed strong bonding with the pancreatic lipase. MD simulations were performed on top five compounds using AMBER16. The simulated complexes revealed stability and ligands remained inside the binding pocket. This study concluded that these finalised molecules can be used as drug candidate to control obesity.
Characterization of leachianone G 2"-dimethylallyltransferase, a novel prenyl side-chain elongation enzyme for the formation of the lavandulyl group of sophoraflavanone G in Sophora flavescens Ait. cell suspension cultures.[Pubmed:14551337]
Plant Physiol. 2003 Nov;133(3):1306-13.
Leachianone G (LG) 2"-dimethylallyltransferase, a novel prenyl side-chain elongation enzyme, was identified in Sophora flavescens Ait. cultured cells. The enzyme transfers a dimethylallyl group to the 2" position of another dimethylallyl group attached at position 8 of LG to form sophoraflavanone G, a branched monoterpenoid-conjugated flavanone characteristic to this plant. This membrane-bound dimethylallyltransferase required Mg2+ (optimum concentration was 10 mm) for the reaction and had an optimum pH of 8.8. It utilized dimethylallyl diphosphate as the sole prenyl donor, and the 2'-hydroxy function in LG was indispensable to the activity. The apparent Km values for dimethylallyl diphosphate and LG were 59 and 2.3 microm, respectively. Subcellular localization of three enzymes that participated in the formation of the lavandulyl group was also investigated by sucrose density gradient centrifugation. Two prenyltransferases, naringenin 8-dimethylallyltransferase and LG 2"-dimethylallyltransferase, were localized in the plastids, whereas 8-dimethylallylnaringenin 2'-hydroxylase, which catalyzes the crucial step in the lavandulyl-group formation, was associated with the endoplasmic reticulum. These results suggest the close cooperation between the plastids and the endoplasmic reticulum in the formation of lavandulyl groups.
Antiviral flavonoids from the root bark of Morus alba L.[Pubmed:12648543]
Phytochemistry. 2003 Apr;62(8):1235-8.
A prenylated flavonoid, moralbanone, along with seven known compounds kuwanon S, mulberroside C, cyclomorusin, eudraflavone B hydroperoxide, oxydihydromorusin, Leachianone G and alpha-acetyl-amyrin were isolated from the root bark of Morus alba L. Leachianone G showed potent antiviral activity (IC(50) = 1.6 microg/ml), whereas mulberroside C showed weak activity (IC(50) = 75.4 microg/ml) against herpes simplex type 1 virus (HSV-1). Their structures were elucidated by spectroscopic methods.
Efficient production and capture of 8-prenylnaringenin and leachianone G-biosynthetic intermediates of sophoraflavanone G--by the addition of cork tissue to cell suspension cultures of Sophora flavescens.[Pubmed:12591262]
Phytochemistry. 2003 Apr;62(7):1093-9.
It has previously been demonstrated that the addition of cork tissue to cell suspension cultures of Sophora flavescens stimulates the production of sophoraflavanone G, most of which has been recovered from the added cork tissue. In the present study, it was found that two precursors of sophoraflavanone G, 8-prenylnaringenin (sophoraflavanone B) and Leachianone G, both of which have never been detected either in cultured cells or in the original plants, also accumulated in the added cork tissue. Thirteen minor flavonoids including three prenylated flavonoids, in addition to 8-prenylnaringenin and Leachianone G, were isolated from the cork tissue co-incubated with S. flavescens cells. The new compounds flavescenones A, B and C, were determined to be (3R)-5, 7, 2'-trihydroxy-6-gamma, gamma-dimethylallyl-4', 5'-methylenedioxyisoflavanone; 5, 7, 2'-trihydroxy-6-gamma, gamma-dimethylallyl-4', 5'-methylenedioxyisoflavone and 2-[2',4'-dihydroxy-3'-(gamma-hydroxymethyl-gamma-methylallyl)phenyl]-5,6-methylen edioxybenzofuran, respectively, by means of spectroscopic analyses that included 2D-NMR techniques.


