A 366G9a/GLP histone lysine methyltransferase inhibitor CAS# 1527503-11-2 |
- KN-92 phosphate
Catalog No.:BCC1682
CAS No.:1135280-28-2
- KN-92 hydrochloride
Catalog No.:BCC1681
CAS No.:1431698-47-3
- Ivermectin
Catalog No.:BCC1251
CAS No.:70288-86-7
- A 438079 hydrochloride
Catalog No.:BCC1317
CAS No.:899431-18-6
- A 438079
Catalog No.:BCC1316
CAS No.:899507-36-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1527503-11-2 | SDF | Download SDF |
| PubChem ID | 76285486 | Appearance | Powder |
| Formula | C19H27N3O2 | M.Wt | 329.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (151.77 mM; Need ultrasonic) | ||
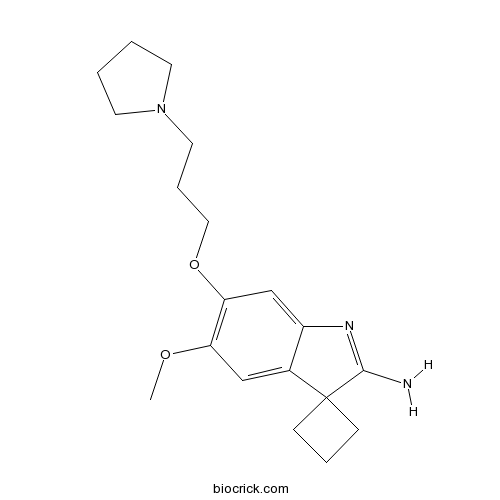
| Chemical Name | 5'-methoxy-6'-(3-pyrrolidin-1-ylpropoxy)spiro[cyclobutane-1,3'-indole]-2'-amine | ||
| SMILES | COC1=C(C=C2C(=C1)C3(CCC3)C(=N2)N)OCCCN4CCCC4 | ||
| Standard InChIKey | BKCDJTRMYWSXMC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H27N3O2/c1-23-16-12-14-15(21-18(20)19(14)6-4-7-19)13-17(16)24-11-5-10-22-8-2-3-9-22/h12-13H,2-11H2,1H3,(H2,20,21) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective G9a/GLP histone lysine methyltransferase inhibitor (IC50 = 3.3 nM). Exhibits >1000-fold selectivity for G9a/GLP over 21 other methyltransferases. Decreases levels of lysine 9 dimethylation on histone H3 (H3K9Me2) in PC3 cells. |

A 366 Dilution Calculator

A 366 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0355 mL | 15.1773 mL | 30.3545 mL | 60.7091 mL | 75.8864 mL |
| 5 mM | 0.6071 mL | 3.0355 mL | 6.0709 mL | 12.1418 mL | 15.1773 mL |
| 10 mM | 0.3035 mL | 1.5177 mL | 3.0355 mL | 6.0709 mL | 7.5886 mL |
| 50 mM | 0.0607 mL | 0.3035 mL | 0.6071 mL | 1.2142 mL | 1.5177 mL |
| 100 mM | 0.0304 mL | 0.1518 mL | 0.3035 mL | 0.6071 mL | 0.7589 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PF-06447475
Catalog No.:BCC5589
CAS No.:1527473-33-1
- Lyconnotine
Catalog No.:BCN1277
CAS No.:6900-93-2
- Cimiside B
Catalog No.:BCN1679
CAS No.:152685-91-1
- Evocarpine
Catalog No.:BCN7064
CAS No.:15266-38-3
- Dihydroevocarpine
Catalog No.:BCN3691
CAS No.:15266-35-0
- 4-Methyl-2-propyl-1H-benzimidazole-6-carboxylic acid
Catalog No.:BCC8713
CAS No.:152628-03-0
- 2-n-Propyl-4-methyl-6-(1-methylbenzimidazole-2-yl)benzimidazole
Catalog No.:BCC8584
CAS No.:152628-02-9
- Testosterone isocaproate
Catalog No.:BCC9170
CAS No.:15262-86-9
- H-Thr(Bzl)-OBzl.oxalate
Catalog No.:BCC3105
CAS No.:15260-11-4
- Boc-Thr(Bzl)-OH
Catalog No.:BCC3451
CAS No.:15260-10-3
- Buddlejasaponin IVb
Catalog No.:BCN2842
CAS No.:152580-79-5
- BMY 45778
Catalog No.:BCC7068
CAS No.:152575-66-1
- Sevelamer HCl
Catalog No.:BCC4718
CAS No.:152751-57-0
- Puerol A
Catalog No.:BCN6566
CAS No.:152784-32-2
- RS 25344 hydrochloride
Catalog No.:BCC7645
CAS No.:152815-28-6
- Chartarlactam A
Catalog No.:BCN7110
CAS No.:1528745-88-1
- Ginkgolide A
Catalog No.:BCN1680
CAS No.:15291-75-5
- Ginkgolide C
Catalog No.:BCN1681
CAS No.:15291-76-6
- Ginkgolide B
Catalog No.:BCN1682
CAS No.:15291-77-7
- Ginkgolide M
Catalog No.:BCC8178
CAS No.:15291-78-8
- Nolatrexed (AG-337)
Catalog No.:BCC6430
CAS No.:152946-68-4
- Dehydro-alpha-lapachone
Catalog No.:BCN1683
CAS No.:15297-92-4
- Guajadial C
Catalog No.:BCN7755
CAS No.:1529775-02-7
- Guajadial D
Catalog No.:BCN7756
CAS No.:1529775-04-9
Pedicled Temporalis Muscle Flap for Craniofacial Reconstruction: A 35-Year Clinical Experience with 366 Flaps.[Pubmed:28121882]
Plast Reconstr Surg. 2017 Feb;139(2):468e-476e.
BACKGROUND: In the past 130 years, the temporalis muscle flap has been used for a variety of different indications. In this age of microsurgery and perforator flaps, the temporalis muscle flap still has many useful applications for craniofacial reconstruction. METHODS: Three hundred sixty-six temporalis muscle flaps were performed in a single center between 1978 and 2012. The authors divided the cases into two series-before and after 1994-because, after 1994, they started to perform free flap reconstructions, and indications for reconstruction with a temporalis muscle flap were changed RESULTS:: In the series after 1994, flaps were most commonly used for reconstruction of defects in the maxilla, mandible, and oropharynx, in addition to facial reanimation and filling of orbital defects. Complications included total flap necrosis (1.6 percent) and partial flap necrosis (10.7 percent). The rate of material extrusion at the donor site decreased after porous polyethylene was uniformly used for reconstruction from 17.1 to 7.9 percent. CONCLUSIONS: The pedicled temporalis muscle flap continues to have many applications in craniofacial reconstruction. With increasing use of free flaps, the authors' indications for the pedicled temporalis muscle flap are now restricted to (1) orbital filling for congenital or acquired anophthalmia; (2) filling of unilateral maxillectomy defects; and (3) facial reanimation in selected cases of facial nerve palsy. CLINICAL QUESTION/LEVEL OF EVIDENCE: Therapeutic, IV.
Discovery and development of potent and selective inhibitors of histone methyltransferase g9a.[Pubmed:24900801]
ACS Med Chem Lett. 2014 Jan 2;5(2):205-9.
G9a is a histone lysine methyltransferase responsible for the methylation of histone H3 lysine 9. The discovery of A-366 arose from a unique diversity screening hit, which was optimized by incorporation of a propyl-pyrrolidine subunit to occupy the enzyme lysine channel. A-366 is a potent inhibitor of G9a (IC50: 3.3 nM) with greater than 1000-fold selectivity over 21 other methyltransferases.


