Imatinib (STI571)Protein-tyrosine kinase inhibitor CAS# 152459-95-5 |
- DCC-2618
Catalog No.:BCC1520
CAS No.:1225278-16-9
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Motesanib
Catalog No.:BCC1776
CAS No.:453562-69-1
- OSI-930
Catalog No.:BCC1253
CAS No.:728033-96-3
- Masitinib (AB1010)
Catalog No.:BCC1260
CAS No.:790299-79-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 152459-95-5 | SDF | Download SDF |
| PubChem ID | 5291 | Appearance | Powder |
| Formula | C29H31N7O | M.Wt | 493.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | STI571 | ||
| Solubility | DMSO : 12.5 mg/mL (25.32 mM; Need ultrasonic) | ||
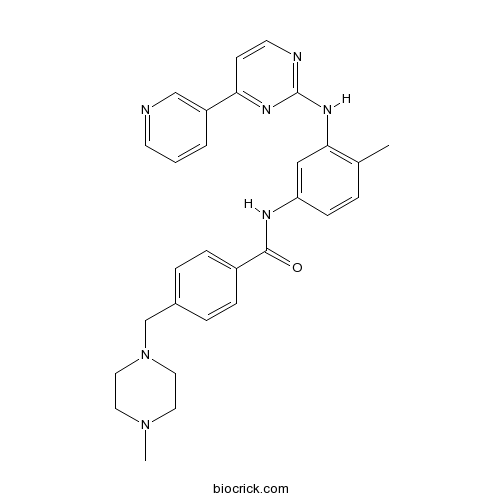
| Chemical Name | 4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide | ||
| SMILES | CC1=C(C=C(C=C1)NC(=O)C2=CC=C(C=C2)CN3CCN(CC3)C)NC4=NC=CC(=N4)C5=CN=CC=C5 | ||
| Standard InChIKey | KTUFNOKKBVMGRW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Imatinib is a selective inhibitor of protein-tyrosine kinase with IC50 values of 0.1, 0.1 and 0.025 μM, respectively against PDGF receptor, c-Kit and Abl. | |||||
| Targets | PDGF receptor | c-Kit | Abl | |||
| IC50 | 0.1 μM | 0.1 μM | 0.025 μM | |||

Imatinib (STI571) Dilution Calculator

Imatinib (STI571) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0259 mL | 10.1297 mL | 20.2593 mL | 40.5186 mL | 50.6483 mL |
| 5 mM | 0.4052 mL | 2.0259 mL | 4.0519 mL | 8.1037 mL | 10.1297 mL |
| 10 mM | 0.2026 mL | 1.013 mL | 2.0259 mL | 4.0519 mL | 5.0648 mL |
| 50 mM | 0.0405 mL | 0.2026 mL | 0.4052 mL | 0.8104 mL | 1.013 mL |
| 100 mM | 0.0203 mL | 0.1013 mL | 0.2026 mL | 0.4052 mL | 0.5065 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Imatinib is an inhibitor of protein-tyrosine kinase with IC50 values of 0.1, 0.1 and 0.025μM, respectively against PDGF receptor, c-Kit and Abl [1].
The type 3 group of receptor tyrosine kinases includes PDGFR, CSF-1R, Flt-3, c-Kit and so on. PDGFRs are found in normal tissues, cells as well as some tumors. It is associated with several nonmalignant proliferative diseases. In vitro assays show that Imatinib can inhibit both PDGF-AA and PDGF-BB stimulated PDGF receptor phosphorylation. Imatinib is also found to inhibit the phosphorylation of c-Kit, another kinase which mediates the growth of a number of tumors. Imatinib inhibits the phosphorylation of these kinases without effecting the expression of them. Some other kinases of the type 3 group (such as Fms and Flt-3) can’t be inhibited by Imatinib, suggesting a selectivity of Imatinib. In addition, Imatinib is shown to significantly inhibit the Bcr-Abl tyrosine kinase both in cell-based assay and in vitro kinase assay. Moreover, as a downstream pathway of PDGF-mediated signals, MAP kinase activation can also be effected in rat A10 smooth muscle cells [1].
References:
[1] Elisabeth Buchdunger, Catherine L. Cioffi, Norman Law, David Stover, Sayuri Ohno-Jones, Brian J. Druker And Nicholas B. Lydon. Abl protein-tyrosine kinase inhibitor sti571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. The journal of pharmacology and experimental therapeutics. 2000, 295(1): 139-145.
- Fmoc-D-Phe(4-OMe)-OH
Catalog No.:BCC2632
CAS No.:152436-04-9
- SAR405
Catalog No.:BCC4006
CAS No.:1523406-39-4
- Gnetulin
Catalog No.:BCN3401
CAS No.:152340-24-4
- (S)-4-(4-Aminobenzyl)-2(1H)-oxazolidinone
Catalog No.:BCC8400
CAS No.:152305-23-2
- Amyloid β-Peptide (10-20) (human)
Catalog No.:BCC1026
CAS No.:152286-31-2
- U 93631
Catalog No.:BCC7471
CAS No.:152273-12-6
- Mupinensisone
Catalog No.:BCN4704
CAS No.:152253-67-3
- Uncargenin C
Catalog No.:BCN1678
CAS No.:152243-70-4
- GT 2016
Catalog No.:BCC7357
CAS No.:152241-24-2
- SB 204741
Catalog No.:BCC7035
CAS No.:152239-46-8
- Aphadilactone C
Catalog No.:BCN7645
CAS No.:1522004-70-1
- Aphadilactone B
Catalog No.:BCN7646
CAS No.:1522004-68-7
- N-(2-Methyl-5-nitrophenyl)-4- (pyridin-3-yl)pyrimidin-2-amine
Catalog No.:BCC9055
CAS No.:152460-09-8
- N-(5-Amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidineamine
Catalog No.:BCC9059
CAS No.:152460-10-1
- Leachianone G
Catalog No.:BCN3308
CAS No.:152464-78-3
- Gnetin J
Catalog No.:BCN3384
CAS No.:152511-23-4
- Nebivolol
Catalog No.:BCC4332
CAS No.:152520-56-4
- PSB 1115
Catalog No.:BCC7237
CAS No.:152529-79-8
- Fmoc-N-Me-Asp(OtBu)-OH
Catalog No.:BCC3212
CAS No.:152548-66-8
- BMY 45778
Catalog No.:BCC7068
CAS No.:152575-66-1
- Buddlejasaponin IVb
Catalog No.:BCN2842
CAS No.:152580-79-5
- Boc-Thr(Bzl)-OH
Catalog No.:BCC3451
CAS No.:15260-10-3
- H-Thr(Bzl)-OBzl.oxalate
Catalog No.:BCC3105
CAS No.:15260-11-4
- Testosterone isocaproate
Catalog No.:BCC9170
CAS No.:15262-86-9
Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS).[Pubmed:20679528]
Blood. 2010 Nov 11;116(19):3758-65.
This study examines the prognostic significance of early molecular response using an expanded dataset in chronic myeloid leukemia patients enrolled in the International Randomized Study of Interferon and STI571 (IRIS). Serial molecular studies demonstrate decreases in BCR-ABL transcripts over time. Analyses of event-free survival (EFS) and time to progression to accelerated phase/blast crisis (AP/BC) at 7 years were based on molecular responses using the international scale (IS) at 6-, 12-, and 18-month landmarks. Patients with BCR-ABL transcripts > 10% at 6 months and > 1% at 12 months had inferior EFS and higher rate of progression to AP/BC compared with all other molecular response groups. Conversely, patients who achieved major molecular response [MMR: BCR-ABL (IS)
A phase I dose-escalation study of imatinib mesylate (Gleevec/STI571) plus capecitabine (Xeloda) in advanced solid tumors.[Pubmed:20530436]
Anticancer Res. 2010 Apr;30(4):1251-6.
UNLABELLED: The aim of this study was to determine the maximally tolerated dose, recommended phase II dose and toxicity profile of capecitabine plus imatinib mesylate combination. PATIENTS AND METHODS: Twenty-four patients with advanced solid tumors were treated with capecitabine twice daily on days 1-14 and imatinib mesylate once daily on a 21-day cycle. Dose-limiting toxicity was assessed during the first cycle. Treatment continued until disease progression or undesirable toxicity. RESULTS: Six patients were treated with capecitabine at 1000 mg/m(2) and imatinib mesylate 300 mg; unacceptable toxicity due to grade 2 intolerable hand-foot syndrome and/or grade > or = 2 diarrhea was observed. Doses were subsequently reduced to capecitabine at 750 mg/m(2) and imatinib mesylate at 300 mg; toxicities were better tolerated at the lower dose. Dose-limiting toxicities consisted of grade 3 diarrhea, anorexia and fatigue lasting > or = 4 days. Treatment-related adverse events greater than or equal to grade 3 included anemia, diarrhea, dysuria, hypophosphatemia and vertigo. Minor responses were observed in two patients: stable disease > or = 6 months was observed in two out of twenty-one evaluable patients. CONCLUSION: Full doses of capecitabine and imatinib mesylate were not tolerable. The maximum tolerated dose and the recommended phase II dose for this drug combination is capecitabine at 750 mg/m(2) twice daily for 1-14 days and imatinib at 300 mg once daily on a 21-day cycle.
Imatinib mesylate (STI571) enhances amrubicin-induced cytotoxic activity through inhibition of the phosphatidylinositol 3-kinase/Akt pathway in small cell lung cancer cells.[Pubmed:19956885]
Oncol Rep. 2010 Jan;23(1):217-22.
Small cell lung cancer (SCLC) is characterized by autocrine mechanisms. Stem cell factor (SCF) and its receptor c-kit can activate Akt and extracellular signal-regulated kinase (Erk) pathways. Imatinib mesylate (STI571) can inhibit c-kit tyrosine kinase activity, but clinical trials have resulted in failure. We investigated the possibility of SCF/c-kit-targeted therapy against SCLC. Using c-kit-positive SCLC cells (H209 and H69 cells) and SCF as a model of the autocrine mechanisms, the effects of SCF, LY294002, PD98059 or STI571 on Akt and Erk were assessed by Western blot analysis. The cell growth inhibitions of cisplatin, etoposide irinotecan and amrubicin (AMR) with or without SCF, LY294002, PD98059 or STI571 were evaluated by MTT assay. Treatment with SCF activated Akt and Erk and the activations were inhibited by STI571 in H209 but not in H69 cells. LY294002 and PD98059 inhibited SCF-induced Akt and Erk activation in H209 cells, respectively. STI571 alone did not exert growth inhibition in the SCF-treated cells. In H209 cells, SCF decreased the cytotoxicity of AMR, but not of other drugs. In H69 cells, SCF did not affect sensitivity to any drugs. LY294002 but not PD98059 restored or enhanced AMR-sensitivity in SCF-treated H209 or untreated H69 cells, respectively. STI571 restored the AMR-sensitivity of SCF-treated H209 cells to the basal level. If the SCF/c-kit contributes to Akt activation in vivo, the combination of STI571 and AMR may be effective against SCLC. Additionally, using a combination of AKT inhibitors and AMR may be a promising treatment in the future.
High rates of durable response are achieved with imatinib after treatment with interferon alpha plus cytarabine: results from the International Randomized Study of Interferon and STI571 (IRIS) trial.[Pubmed:19648168]
Haematologica. 2009 Dec;94(12):1669-75.
BACKGROUND: Imatinib is the standard of care for newly diagnosed chronic-phase chronic myeloid leukemia. The largest randomized clinical trial of imatinib was the multinational IRIS trial in which 1106 patients were randomized to receive either imatinib 400 mg/day or a standard regimen of interferon-alpha plus cytarabine. DESIGN AND METHODS: Patients were allowed to cross over to the opposite treatment for intolerance, lack of response, disease progression, and, following release of the initial efficacy data, reluctance to remain on therapy with interferon-alpha plus cytarabine. The safety and efficacy of imatinib in patients who crossed over from interferon-alpha plus cytarabine to imatinib is reported here. RESULTS: Of 553 patients originally assigned to interferon-alpha plus cytarabine, 65% crossed over to imatinib, of whom 67% continue to receive treatment. After a median of 54 months of imatinib treatment on study, 93% achieved complete hematologic remission, 86% achieved major cytogenetic remission, and 81% achieved a complete cytogenetic remission as the best observed response. Estimated rates of freedom from progression to accelerated or blast phase and overall survival were 91% and 89%, respectively, at 48 months after starting imatinib. CONCLUSIONS: This is the largest analysis to date describing the efficacy of imatinib in patients who have received prior therapies for chronic myeloid leukemia and it demonstrates excellent responses to this treatment. (ClinicalTrials.gov identifier: NCT00006343).


