AristoloneCAS# 6831-17-0 |
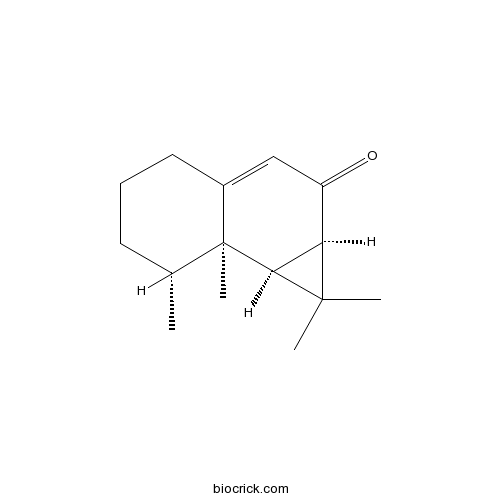
- Aristol-9-en-8-one
Catalog No.:BCN9166
CAS No.:25274-27-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6831-17-0 | SDF | Download SDF |
| PubChem ID | 165536 | Appearance | Powder |
| Formula | C15H22O | M.Wt | 218.3 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Synonyms | 25274-27-5 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1aR,7R,7aR,7bS)-1,1,7,7a-tetramethyl-1a,4,5,6,7,7b-hexahydrocyclopropa[a]naphthalen-2-one | ||
| SMILES | CC1CCCC2=CC(=O)C3C(C12C)C3(C)C | ||
| Standard InChIKey | UGVIZCBJCSXBCJ-JWFUOXDNSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Aristolone is a natural product from the leaf oil of Alpinia polyantha. |
| In vitro | New bioactive secondary metabolites from Bornean red alga, Laurencia similis (Ceramiales).[Pubmed: 23678792]Nat Prod Commun. 2013 Mar;8(3):287-8.
|
| Structure Identification | Nat Prod Commun. 2015 Feb;10(2):367-8.Chemical constituents of essential oils from the leaves, stems, roots and fruits of Alpinia polyantha.[Pubmed: 25920286]The essential oils obtained from the leaves, stems, roots and fruits of Alpinia polyantha D. Fang (Zingiberaceae) have been studied. |

Aristolone Dilution Calculator

Aristolone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5809 mL | 22.9043 mL | 45.8085 mL | 91.617 mL | 114.5213 mL |
| 5 mM | 0.9162 mL | 4.5809 mL | 9.1617 mL | 18.3234 mL | 22.9043 mL |
| 10 mM | 0.4581 mL | 2.2904 mL | 4.5809 mL | 9.1617 mL | 11.4521 mL |
| 50 mM | 0.0916 mL | 0.4581 mL | 0.9162 mL | 1.8323 mL | 2.2904 mL |
| 100 mM | 0.0458 mL | 0.229 mL | 0.4581 mL | 0.9162 mL | 1.1452 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Amlexanox
Catalog No.:BCC8817
CAS No.:68302-57-8
- Amlexanox ethyl ester
Catalog No.:BCC8818
CAS No.:68301-99-5
- Planinin
Catalog No.:BCC8981
CAS No.:68296-27-5
- Zonisamide sodium
Catalog No.:BCC4240
CAS No.:68291-98-5
- Zonisamide
Catalog No.:BCC2512
CAS No.:68291-97-4
- Boc-Arg(Mts)-OH.CHA
Catalog No.:BCC3045
CAS No.:68262-71-5
- (R)-Oxiracetam
Catalog No.:BCC4169
CAS No.:68252-28-8
- 2-Hydroxy-3-(hydroxymethyl)anthraquinone
Catalog No.:BCN1380
CAS No.:68243-30-1
- 6-Prenylnaringenin
Catalog No.:BCN2999
CAS No.:68236-13-5
- 6,8-Diprenylnaringenin
Catalog No.:BCN3000
CAS No.:68236-11-3
- BRL 44408 maleate
Catalog No.:BCC6948
CAS No.:681806-46-2
- Isoliensinine
Catalog No.:BCN6331
CAS No.:6817-41-0
- Imbricatolic acid
Catalog No.:BCN4241
CAS No.:6832-60-6
- Nonactin
Catalog No.:BCC7388
CAS No.:6833-84-7
- 1-Methyl-2-nonylquinolin-4(1H)-one
Catalog No.:BCN6585
CAS No.:68353-24-2
- 23,24-dihydroisocucurbitacin B
Catalog No.:BCN7876
CAS No.:68354-21-2
- Deltaline
Catalog No.:BCN5404
CAS No.:6836-11-9
- 7-Methoxy-1-tetralone
Catalog No.:BCN2241
CAS No.:6836-19-7
- 7-Methoxy-1-naphthaleneacetic acid ethyl ester
Catalog No.:BCN1379
CAS No.:6836-21-1
- 7-Methoxy-1-naphthaleneacetic acid
Catalog No.:BCN2243
CAS No.:6836-22-2
- Micheliolide
Catalog No.:BCN8257
CAS No.:68370-47-8
- Sulbactam
Catalog No.:BCC4941
CAS No.:68373-14-8
- JNJ 5207787
Catalog No.:BCC6100
CAS No.:683746-68-1
- Curassavine
Catalog No.:BCN1964
CAS No.:68385-70-6
Chemical constituents of essential oils from the leaves, stems, roots and fruits of Alpinia polyantha.[Pubmed:25920286]
Nat Prod Commun. 2015 Feb;10(2):367-8.
The essential oils obtained from the leaves, stems, roots and fruits of Alpinia polyantha D. Fang (Zingiberaceae) have been studied. The leaf oil was comprised mainly of camphor (16.1%), alpha-pinene (15.2%) and beta-agarofuran (12.9%), while the major constituents of the stem oil were alpha-pinene (12.4%), beta-cubebene (10.6%), beta-agarofuran (10.3%) and globulol (8.8%). However, beta-cubebene (12.6%), fenchyl acetate (10.8%), beta-maaliene (9.0%), Aristolone (8.8%) and alpha-pinene (8.2%) were the compounds occurring in higher amounts in the root oil. The quantitatively significant compounds of the fruit oil were delta-cadinene (10.9%), beta-caryophyllene (9.1%), beta-pinene (8.7%) and alpha-muurolene (7.7%).
Inhibitory effects of wild dietary plants on lipid peroxidation and on the proliferation of human cancer cells.[Pubmed:26408343]
Food Chem Toxicol. 2015 Dec;86:16-24.
Thirteen hydroalcoholic extracts of edible plants from Southern Italy were evaluated for their in vitro antioxidant and antiproliferative activity on three human cancer cell lines: breast cancer MCF-7, hepatic cancer HepG2 and colorectal cancer LoVo. After 48 h of incubation the most antiproliferative plant extract was rosemary (Rosmarinus officinalis L.) on LoVo cell line with IC50 of 16.60 microg/ml. Oregano (Origanum vulgare L. subsp. viridulum) showed a selective antiproliferative activity on hepatic cancer with IC50 of 32.59 microg/ml. All the extracts, with the exception of Diplotaxis tenuifolia (L.) DC., exerted antioxidant properties, the most active plants being dewberry (Rubus caesius L.) and "laprista" (Rumex conglomerates Murray) with IC50 of 4.91 and 5.53 microg/ml, respectively. Rumex conglomeratus contained the highest amount of flavonoids (15.5 mg/g) followed by Portulaca oleracea L. (11.8 mg/g). Rosmarinus officinalis contained the highest number of terpenes. Among them ketoursene (14.7%) and Aristolone (11.3%) were found to be the major constituents. P. oleracea and Raphanus raphanistrum L. subsp. landra contained the highest number of sterols.
Optimization of the extraction of the p-menthadienol isomers and aristolone contained in the essential oil from Elyonurus hensii using a 2(3) full factorial design.[Pubmed:28572969]
Food Sci Nutr. 2017 Feb 9;5(3):784-792.
The aim of this study was to optimize the extraction of p-menthadienol isomers and Aristolone from the essential oil of Elyonurus hensii by hydrodistillation. The study of the seasonal variation in the chemical composition has shown that the plant material has been subject to a natural selection regarding the biosynthesis of the p-menthadienol isomers: during periods of water stress, the extracts are rich in cis and trans-p-mentha-1(7),8-dien-2-ol and poor in cis and trans-p-mentha-2,8-dien-1-ol. Regarding the modeling, eight experiments were carried out by considering three easily interpretable factors (the extraction duration, the residual water content and the state of the division of the plant material). The average yield was 1.33% for the aerial part and 0.74% for the roots. The residual water content is the most important factor, which significantly influences the average yield of the essential oil and the content of the major constituents. Regarding the aerial part, a low residual water content of the plant material varies the essential oil yield (from 0.40% to 2.11%) and the content of cis and trans-p-mentha-2.8-dien-1-ol (from 15.87% to 23.24%). At the root level, the samples that have a very low residual water content provide extracts richer in Aristolone. The combined effects of the extraction duration, the state of division, and the residual water content influence greatly the extraction of Aristolone (from 36.68% to 54.55%). However, these interactions are more complex and difficult to assess.
A Simple and Rapid UPLC-PDA Method for Quality Control of Nardostachys jatamansi.[Pubmed:29202512]
Planta Med. 2018 May;84(8):536-543.
Nardostachys jatamansi is a well-documented herbal agent used to treat digestive and neuropsychiatric disorders in oriental medicinal systems. However, few simple, rapid, and comprehensive methods were reported for quality assessment and control of N. jatamansi. Herein, a UPLC with photodiode array detection method was developed for both fingerprint investigation of N. jatamansi and simultaneous quantitative analysis of the six serotonin transporter modulatory constituents in N. jatamansi. For chromatographic fingerprinting, 24 common peaks were selected as characteristic peaks to assess the consistency of N. jatamansi samples from different retail sources. Six of the common peaks (5, 7, 12: , and 16: - 18: ) were identified as desoxo-narchinol A, buddleoside, isonardosinone, nardosinone, kanshone H, and (-)-Aristolone, respectively, by phytochemical investigation. Five of the six compounds significantly either enhanced or inhibited serotonin transporter activity, while (-)-Aristolone (18: ) didn't show any serotonin transporter activity. In quantitative analysis, the six compounds showed good linearity (r > 0.999) within test ranges. The precision, expressed as relative standard deviation, was in the range of 0.25 - 2.77%, and the recovery of the method was in the range of 92 - 105%. The UPLC-photodiode array detection-based fingerprint analysis and quantitative methods reported here could be used for routine quality control of N. jatamansi.
Evaluation of the cytotoxic effects of Cyperus longus extract, fractions and its essential oil on the PC3 and MCF7 cancer cell lines.[Pubmed:26893742]
Oncol Lett. 2016 Feb;11(2):1353-1360.
Cyperus longus is one of the Iranian endemic species. However, to date, and to the best of our knowledge, there are no availale academic reports on the cytotoxicity of this plant. Thus, this study was carried out to examine the in vitro anti-proliferative and anti-apoptotic effects of Cyperus longus extract, fractions and essential oil (EO) on MCF7 and PC3 cell lines. The chemical constituents of EO were identified using gas chromatography (GC)-mass spectrometry (MS) analysis. The cells were cultured in RPMI-1640 medium and incubated with various concentrations of the plant extract and fractions. Cell viability was quantified by MTT assay following 24, 48 and 72 h of exposure to (12.5-200 microg/ml) of the methanol extract, the dichloromethane (CH2Cl2), ethyl acetate (EtOAc) and water fractions, as well as the EO of the plant. The percentage of apoptotic cells was determined using propidium iodide staining of DNA fragments by flow cytometry (sub-G1 peak). The most effective fraction in the MCF7 cell line was the CH2Cl2 fraction (IC50 after 48 h, 25.34+/-2.01). The EtOAc fraction (IC50 after 48 h, 35.2+/-2.69) and the methanol extract (IC50 after 48 h, 64.64+/-1.64) were also found to be effective. The IC50 values obtained for the PC3 cell line were 37.97+/-3.87, 51.57+/-3.87 and 70.33+/-2.36 for the CH2Cl2 fraction, the EtOAc fraction and the methanol extract, respectively. Based on these data and due to the partial polarity of the most effective fraction (the CH2Cl2 fraction), we also examined the cytotoxicity of the plant EO. The IC50 values after 48 h were 22.25+/-4.25 and 12.55+/-3.65 in the PC3 and MCF7 cell lines, respectively. DNA fragmentation assay also confirmed these data. Performing GC-MS analysis for the plant EO revealed that beta-himachalene (10.81%), alpha-caryophyllene oxide (7.6%), irisone (4.78%), beta-caryophyllene oxide (4.36%), humulene oxide (12%), viridiflorol (4.73%), Aristolone (6.39%) and longiverbenone (6.04%) were the main constituents. Our results demonstrated that two of the constituents of Cyperus longus, viridiflorol and longiverbenone, should be investigated further as possible promising chemotherapeutic agents in cancer treatment.
Terpenoids from Russula lepida and R. amarissima (Basidiomycota, Russulaceae).[Pubmed:22938994]
Phytochemistry. 2012 Dec;84:154-9.
Four aristolane sesquiterpenes were isolated from the fruiting bodies of Russula lepida and R. amarissima, namely (1R,2S)-1,2-dihydroxyAristolone (6), (2S,11S)-2,12-dihydroxy-Aristolone (7), (1R,2S,11S)-1,2,12-trihydroxyAristolone (8), (1S,2S,11S)-1,2,12-trihydroxy-Aristolone (9). In addition, a seco-cucurbitane triterpene, i.e. 3,4-secocucurbita-4,24E-diene-3-hydroxy-26-carboxylic acid (14) was isolated from both species. The configuration at C-2 of the already known rulepidol (2-hydroxyAristolone, 5) was corrected as S instead of R. Several more aristolane and nardosinane sesquiterpenes, as well as cucurbitane triterpenes, already reported both from European and Chinese samples of R. lepida, were also isolated. Compound 14 showed moderate cell growth inhibitory activity.
New bioactive secondary metabolites from Bornean red alga, Laurencia similis (Ceramiales).[Pubmed:23678792]
Nat Prod Commun. 2013 Mar;8(3):287-8.
A Bomean red algal population of Laurencia similis Nam et Saito was analyzed for its secondary metabolite composition. Seven compounds were identified: ent-1(10)-aristolen-9beta-ol (1), (+)-Aristolone (2), axinysone B (3), 9-aristolen-1alpha-ol (4), 2,3,5,6-tetrabromoindole (5), 1-methyl-2,3,5,6-tetrabromoindole (6), and 1-methyl-2,3,5-tribromoindole (7). Compound 1 was identified as a new optical isomer of 1(10)-aristolen-9beta-ol. Compounds 1, 4 and 5 exhibited good antibacterial activity against antibiotic resistant clinical bacteria and cytotoxic effects against selected cancer cell lines.


