Aristol-9-en-8-oneCAS# 25274-27-5 |
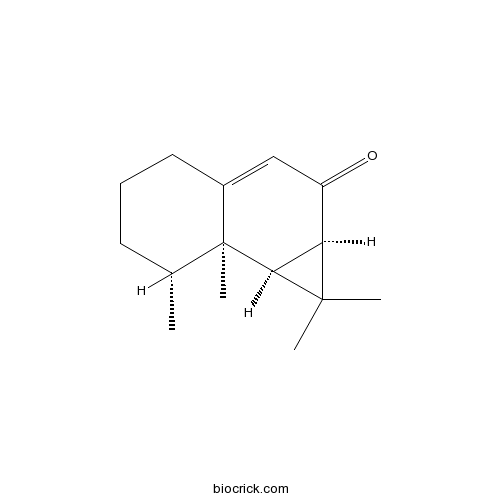
- Aristolone
Catalog No.:BCN4039
CAS No.:6831-17-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 25274-27-5 | SDF | Download SDF |
| PubChem ID | 165536 | Appearance | Powder |
| Formula | C15H22O | M.Wt | 218.33 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Synonyms | 6831-17-0 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1aR,7R,7aR,7bS)-1,1,7,7a-tetramethyl-1a,4,5,6,7,7b-hexahydrocyclopropa[a]naphthalen-2-one | ||
| SMILES | CC1CCCC2=CC(=O)C3C(C12C)C3(C)C | ||
| Standard InChIKey | UGVIZCBJCSXBCJ-JWFUOXDNSA-N | ||
| Standard InChI | InChI=1S/C15H22O/c1-9-6-5-7-10-8-11(16)12-13(14(12,2)3)15(9,10)4/h8-9,12-13H,5-7H2,1-4H3/t9-,12-,13+,15+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Aristol-9-en-8-one Dilution Calculator

Aristol-9-en-8-one Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5802 mL | 22.9011 mL | 45.8022 mL | 91.6045 mL | 114.5056 mL |
| 5 mM | 0.916 mL | 4.5802 mL | 9.1604 mL | 18.3209 mL | 22.9011 mL |
| 10 mM | 0.458 mL | 2.2901 mL | 4.5802 mL | 9.1604 mL | 11.4506 mL |
| 50 mM | 0.0916 mL | 0.458 mL | 0.916 mL | 1.8321 mL | 2.2901 mL |
| 100 mM | 0.0458 mL | 0.229 mL | 0.458 mL | 0.916 mL | 1.1451 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-Ethoxy-3-acetonyltaxifolin
Catalog No.:BCN9165
CAS No.:2212305-01-4
- 4,18-Dihydro-4-hydroxysaprirearine
Catalog No.:BCN9164
CAS No.:2202760-88-9
- Prionidipene A
Catalog No.:BCN9163
CAS No.:2199455-72-4
- 6-Hydroxycoumurrayin
Catalog No.:BCN9162
CAS No.:2188162-97-0
- (11Bs)-7,11-dihydroxy-3,4,9,11b-tetramethyl-1,2,8,9-tetrahydronaphtho[2,1-f][1]benzofuran-6-one
Catalog No.:BCN9161
CAS No.:
- Soyasapogenol A 21-O-α-L-rhamnopyranoside
Catalog No.:BCN9160
CAS No.:2067321-88-2
-
(-)-Evofolin B
Catalog No.:BCN9159
CAS No.:1961305-60-1
- Glabralide A
Catalog No.:BCN9158
CAS No.:1969289-10-8
- Fuscaxanthone A
Catalog No.:BCN9157
CAS No.:499777-91-2
- Myricadenin A
Catalog No.:BCN9156
CAS No.:1612239-23-2
- Glabralide C
Catalog No.:BCN9155
CAS No.:2170388-85-7
- Glabralide B
Catalog No.:BCN9154
CAS No.:2170388-84-6
- 7-O-Methylepimedonin G
Catalog No.:BCN9167
CAS No.:2220243-40-1
- Ethyl β-D-apiofuranoside
Catalog No.:BCN9168
CAS No.:1932405-61-2
- Epimedonin I
Catalog No.:BCN9169
CAS No.:2222285-83-6
- Epimedonin J
Catalog No.:BCN9170
CAS No.:2222285-84-7
- Epimedonin K
Catalog No.:BCN9171
CAS No.:2222285-85-8
- Epimedonin G
Catalog No.:BCN9172
CAS No.:2222285-80-3
- Epimedonin H
Catalog No.:BCN9173
CAS No.:2222285-82-5
- Tinosporol B
Catalog No.:BCN9174
CAS No.:2244777-13-5
- Tinosporol A
Catalog No.:BCN9175
CAS No.:2244777-12-4
- Tinosporoside A
Catalog No.:BCN9176
CAS No.:2244777-15-7
- Tinosporol C
Catalog No.:BCN9177
CAS No.:2244777-14-6
- Dehydroevodiamine hydrochloride
Catalog No.:BCN9178
CAS No.:111664-82-5
Aristolane-type Sesquiterpenoids from Nardostachys chinensis and Revised Structure of Aristolanhydride.[Pubmed:30848431]
Nat Prod Bioprospect. 2019 Apr;9(2):149-155.
Four hitherto unknown aristolane-type sesquiterpenes, including one novel 8,9-secoaristolane, namely secoaristolenedioic acid (1), two aristolone derivatives, namely 1alpha,2beta-dihydroxyaristolone (2), 9-epidebilon (3), and one rare aristolane-chalcone hybrid, namely 3'-hydroxynardoaristolone A (4) were isolated from the ethanol extract of the roots and rhizomes of Nardostachys chinensis. Their structures were elucidated on the basis of extensive spectroscopic analysis. In addition, the structure of aristolanhydride, recently isolated from the same species, was corrected by reanalysis of the published NMR data.
A Simple and Rapid UPLC-PDA Method for Quality Control of Nardostachys jatamansi.[Pubmed:29202512]
Planta Med. 2018 May;84(8):536-543.
Nardostachys jatamansi is a well-documented herbal agent used to treat digestive and neuropsychiatric disorders in oriental medicinal systems. However, few simple, rapid, and comprehensive methods were reported for quality assessment and control of N. jatamansi. Herein, a UPLC with photodiode array detection method was developed for both fingerprint investigation of N. jatamansi and simultaneous quantitative analysis of the six serotonin transporter modulatory constituents in N. jatamansi. For chromatographic fingerprinting, 24 common peaks were selected as characteristic peaks to assess the consistency of N. jatamansi samples from different retail sources. Six of the common peaks (5, 7, 12: , and 16: - 18: ) were identified as desoxo-narchinol A, buddleoside, isonardosinone, nardosinone, kanshone H, and (-)-aristolone, respectively, by phytochemical investigation. Five of the six compounds significantly either enhanced or inhibited serotonin transporter activity, while (-)-aristolone (18: ) didn't show any serotonin transporter activity. In quantitative analysis, the six compounds showed good linearity (r > 0.999) within test ranges. The precision, expressed as relative standard deviation, was in the range of 0.25 - 2.77%, and the recovery of the method was in the range of 92 - 105%. The UPLC-photodiode array detection-based fingerprint analysis and quantitative methods reported here could be used for routine quality control of N. jatamansi.
Optimization of the extraction of the p-menthadienol isomers and aristolone contained in the essential oil from Elyonurus hensii using a 2(3) full factorial design.[Pubmed:28572969]
Food Sci Nutr. 2017 Feb 9;5(3):784-792.
The aim of this study was to optimize the extraction of p-menthadienol isomers and aristolone from the essential oil of Elyonurus hensii by hydrodistillation. The study of the seasonal variation in the chemical composition has shown that the plant material has been subject to a natural selection regarding the biosynthesis of the p-menthadienol isomers: during periods of water stress, the extracts are rich in cis and trans-p-mentha-1(7),8-dien-2-ol and poor in cis and trans-p-mentha-2,8-dien-1-ol. Regarding the modeling, eight experiments were carried out by considering three easily interpretable factors (the extraction duration, the residual water content and the state of the division of the plant material). The average yield was 1.33% for the aerial part and 0.74% for the roots. The residual water content is the most important factor, which significantly influences the average yield of the essential oil and the content of the major constituents. Regarding the aerial part, a low residual water content of the plant material varies the essential oil yield (from 0.40% to 2.11%) and the content of cis and trans-p-mentha-2.8-dien-1-ol (from 15.87% to 23.24%). At the root level, the samples that have a very low residual water content provide extracts richer in aristolone. The combined effects of the extraction duration, the state of division, and the residual water content influence greatly the extraction of aristolone (from 36.68% to 54.55%). However, these interactions are more complex and difficult to assess.
Antidiabetic potentials of ethanolic extract of Aristolochia ringens (Vahl.) roots.[Pubmed:26899440]
J Ethnopharmacol. 2016 Apr 22;182:122-8.
ETHNOPHARMACOLOGICAL IMPORTANCE: Oral administration of alcoholic decoctions of Aristolochia ringens is used extensively by Traditional Medicine Practitioners (TMP) of Yoruba heritage in South west, Nigeria for the management of diabetes and its associated complications. AIM OF THE STUDY: To assess the potentials of root ethanolic extract of Aristolochia ringens V. (REAR) in the management of diabetes using chemical induced experimental animal model. MATERIALS AND METHODS: Qualitative and quantitative analyses were carried out to elucidate chemical constituents of the REAR extract using phytochemical analyses and gas chromatography-mass spectrometry (GC-MS) technique. Streptozotocin (STZ)-induced diabetic rats were orally administered with 75, 150 and 300mg/kg body weight of the REAR, once a day and the blood glucose (GLU) levels were monitored for 14 days. Mechanisms of GLU lowering effect were investigated by performing oral glucose tolerance test (OGTT) and modulation of diabetes associated biomarkers including hepatic glycogen (GLY), GLU, alpha amylase (alphaAMY) and glucose-6-phosphate dehydrogenase (GPDH) by the extract. RESULTS: Extraction from A. ringens roots using ethanol/water (70/30) yielded 10.25% w/w REAR extract. Preliminary phytochemical profiling of REAR extract revealed the presence of flavonoids (23.03%), phenols (19.15%), alkaloids (16.13%), tannins (4.21%), saponins (1.44%) and GC-MS analyses showed that bulk of the extract was constituted by aristolone (92.3%). Oral administration of 300mg/kg b. wt. REAR extract caused significant reduction (>90%) in hyperglycemia to normal GLU level (
Evaluation of the cytotoxic effects of Cyperus longus extract, fractions and its essential oil on the PC3 and MCF7 cancer cell lines.[Pubmed:26893742]
Oncol Lett. 2016 Feb;11(2):1353-1360.
Cyperus longus is one of the Iranian endemic species. However, to date, and to the best of our knowledge, there are no availale academic reports on the cytotoxicity of this plant. Thus, this study was carried out to examine the in vitro anti-proliferative and anti-apoptotic effects of Cyperus longus extract, fractions and essential oil (EO) on MCF7 and PC3 cell lines. The chemical constituents of EO were identified using gas chromatography (GC)-mass spectrometry (MS) analysis. The cells were cultured in RPMI-1640 medium and incubated with various concentrations of the plant extract and fractions. Cell viability was quantified by MTT assay following 24, 48 and 72 h of exposure to (12.5-200 microg/ml) of the methanol extract, the dichloromethane (CH2Cl2), ethyl acetate (EtOAc) and water fractions, as well as the EO of the plant. The percentage of apoptotic cells was determined using propidium iodide staining of DNA fragments by flow cytometry (sub-G1 peak). The most effective fraction in the MCF7 cell line was the CH2Cl2 fraction (IC50 after 48 h, 25.34+/-2.01). The EtOAc fraction (IC50 after 48 h, 35.2+/-2.69) and the methanol extract (IC50 after 48 h, 64.64+/-1.64) were also found to be effective. The IC50 values obtained for the PC3 cell line were 37.97+/-3.87, 51.57+/-3.87 and 70.33+/-2.36 for the CH2Cl2 fraction, the EtOAc fraction and the methanol extract, respectively. Based on these data and due to the partial polarity of the most effective fraction (the CH2Cl2 fraction), we also examined the cytotoxicity of the plant EO. The IC50 values after 48 h were 22.25+/-4.25 and 12.55+/-3.65 in the PC3 and MCF7 cell lines, respectively. DNA fragmentation assay also confirmed these data. Performing GC-MS analysis for the plant EO revealed that beta-himachalene (10.81%), alpha-caryophyllene oxide (7.6%), irisone (4.78%), beta-caryophyllene oxide (4.36%), humulene oxide (12%), viridiflorol (4.73%), aristolone (6.39%) and longiverbenone (6.04%) were the main constituents. Our results demonstrated that two of the constituents of Cyperus longus, viridiflorol and longiverbenone, should be investigated further as possible promising chemotherapeutic agents in cancer treatment.
Inhibitory effects of wild dietary plants on lipid peroxidation and on the proliferation of human cancer cells.[Pubmed:26408343]
Food Chem Toxicol. 2015 Dec;86:16-24.
Thirteen hydroalcoholic extracts of edible plants from Southern Italy were evaluated for their in vitro antioxidant and antiproliferative activity on three human cancer cell lines: breast cancer MCF-7, hepatic cancer HepG2 and colorectal cancer LoVo. After 48 h of incubation the most antiproliferative plant extract was rosemary (Rosmarinus officinalis L.) on LoVo cell line with IC50 of 16.60 microg/ml. Oregano (Origanum vulgare L. subsp. viridulum) showed a selective antiproliferative activity on hepatic cancer with IC50 of 32.59 microg/ml. All the extracts, with the exception of Diplotaxis tenuifolia (L.) DC., exerted antioxidant properties, the most active plants being dewberry (Rubus caesius L.) and "laprista" (Rumex conglomerates Murray) with IC50 of 4.91 and 5.53 microg/ml, respectively. Rumex conglomeratus contained the highest amount of flavonoids (15.5 mg/g) followed by Portulaca oleracea L. (11.8 mg/g). Rosmarinus officinalis contained the highest number of terpenes. Among them ketoursene (14.7%) and aristolone (11.3%) were found to be the major constituents. P. oleracea and Raphanus raphanistrum L. subsp. landra contained the highest number of sterols.
Chemical constituents of essential oils from the leaves, stems, roots and fruits of Alpinia polyantha.[Pubmed:25920286]
Nat Prod Commun. 2015 Feb;10(2):367-8.
The essential oils obtained from the leaves, stems, roots and fruits of Alpinia polyantha D. Fang (Zingiberaceae) have been studied. The leaf oil was comprised mainly of camphor (16.1%), alpha-pinene (15.2%) and beta-agarofuran (12.9%), while the major constituents of the stem oil were alpha-pinene (12.4%), beta-cubebene (10.6%), beta-agarofuran (10.3%) and globulol (8.8%). However, beta-cubebene (12.6%), fenchyl acetate (10.8%), beta-maaliene (9.0%), aristolone (8.8%) and alpha-pinene (8.2%) were the compounds occurring in higher amounts in the root oil. The quantitatively significant compounds of the fruit oil were delta-cadinene (10.9%), beta-caryophyllene (9.1%), beta-pinene (8.7%) and alpha-muurolene (7.7%).
New bioactive secondary metabolites from Bornean red alga, Laurencia similis (Ceramiales).[Pubmed:23678792]
Nat Prod Commun. 2013 Mar;8(3):287-8.
A Bomean red algal population of Laurencia similis Nam et Saito was analyzed for its secondary metabolite composition. Seven compounds were identified: ent-1(10)-aristolen-9beta-ol (1), (+)-aristolone (2), axinysone B (3), 9-aristolen-1alpha-ol (4), 2,3,5,6-tetrabromoindole (5), 1-methyl-2,3,5,6-tetrabromoindole (6), and 1-methyl-2,3,5-tribromoindole (7). Compound 1 was identified as a new optical isomer of 1(10)-aristolen-9beta-ol. Compounds 1, 4 and 5 exhibited good antibacterial activity against antibiotic resistant clinical bacteria and cytotoxic effects against selected cancer cell lines.
Terpenoids from Russula lepida and R. amarissima (Basidiomycota, Russulaceae).[Pubmed:22938994]
Phytochemistry. 2012 Dec;84:154-9.
Four aristolane sesquiterpenes were isolated from the fruiting bodies of Russula lepida and R. amarissima, namely (1R,2S)-1,2-dihydroxyaristolone (6), (2S,11S)-2,12-dihydroxy-aristolone (7), (1R,2S,11S)-1,2,12-trihydroxyaristolone (8), (1S,2S,11S)-1,2,12-trihydroxy-aristolone (9). In addition, a seco-cucurbitane triterpene, i.e. 3,4-secocucurbita-4,24E-diene-3-hydroxy-26-carboxylic acid (14) was isolated from both species. The configuration at C-2 of the already known rulepidol (2-hydroxyaristolone, 5) was corrected as S instead of R. Several more aristolane and nardosinane sesquiterpenes, as well as cucurbitane triterpenes, already reported both from European and Chinese samples of R. lepida, were also isolated. Compound 14 showed moderate cell growth inhibitory activity.
HPTLC and reverse phase HPLC methods for the simultaneous quantification and in vitro screening of antioxidant potential of isolated sesquiterpenoids from the rhizomes of Cyperus rotundus.[Pubmed:22877740]
J Chromatogr B Analyt Technol Biomed Life Sci. 2012 Sep 1;904:22-8.
Three sesquiterpenoids solavetivone, aristolone and nootkatone were isolated from the acetone extract of Cyperus rotundus by silica gel column chromatography and identified by spectral studies. Solavetivone has been isolated for the first time from the species. Simple, sensitive and selective HPTLC and HPLC methods with ultraviolet detection (245 nm) were developed and validated for the simultaneous quantification. HPTLC method was validated in terms of their linearity, LOD, LOQ, precision, accuracy and compared with RP-HPLC-UV method. Among the three sesquiterpenoids isolated, nootkatone possessed the highest radical scavenging potential (IC(50) 4.81 mug/ml) followed by aristolone (IC(50) 5.28 mug/ml) and solavetivone (IC(50) 6.82 mug/ml) by DPPH radical scavenging assay. Total antioxidant activity against phosphomolybdenum reagent was also studied. The methods described in this paper were able to identify and quantify sesquiterpenoids from the complex mixtures of phytochemicals and could be extended to the marker based standardization of polyherbal formulations containing C. rotundus.
Disesquiterpene and sesquiterpene coumarins from Ferula pseudalliacea, and determination of their absolute configurations.[Pubmed:22440297]
Phytochemistry. 2012 Jun;78:170-8.
The first disesquiterpene coumarin, sanandajin, five sesquiterpene coumarins, kamolonol acetate, fekrynol acetate, ethyl galbanate, methyl galbanate, farnesiferol B, and a sesquiterpene, aristolone, were isolated from a n-hexane extract of Ferula pseudalliacea roots. The structures were elucidated by 1D and 2D NMR, HR-ESIMS data, and kamolonol acetate was confirmed by single-crystal X-ray analysis. The absolute configuration of compounds was established by comparison of experimental and simulated ECD spectra using time dependence density function theory (TDDFT). In vitro antiplasmodial activity against Plasmodium falciparum K1 strain was determined. sanandajin, kamolonol acetate and methyl galbanate showed moderate antiplasmodial activity, with IC(50) values of 2.6, 16.1 and 7.1 muM, respectively.
A novel brominated cuparene-derived sesquiterpene ether from the red alga Laurencia sp.[Pubmed:20924905]
J Asian Nat Prod Res. 2010 Oct;12(10):916-20.
A novel brominated cuparene-derived sesquiterpene ether, 8,10-dibromo-3,7-epoxy-laur-13-ol (1), was isolated from Laurencia sp. collected in South China Sea. Besides this, two known sesquiterpenes, (9beta)-aristol-1(10)-en-9-ol (2) and aristolone (3), were also yielded, and aristolone (3) was obtained from Laurencia for the first time. Their structures were elucidated by spectroscopic methods.
Chemical analysis and biological activity of the essential oils of two valerianaceous species from China: Nardostachys chinensis and Valeriana officinalis.[Pubmed:20877232]
Molecules. 2010 Sep 14;15(9):6411-22.
In order to investigate essential oils with biological activity from local wild plants, two valerianaceous species, Nardostachys chinensis and Valeriana officinalis, were screened for their antimicrobial and antioxidant activity. The essential oils were obtained from the roots and rhizomes of the two plants by hydro-distillation, and were analyzed for their chemical composition by gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS). Calarene (25.31%), aristolone (13.35%), alpha-selinene (7.32%) and beta-maaliene (6.70%) were the major compounds of the 23 identified components which accounted for 92.76% of the total oil of N. chinensis. Patchoulol (16.75%), alpha-pinene (14.81%), and beta-humulene (8.19%) were the major compounds among the 20 identified components, which accounted for 88.11% of the total oil of V. officinalis. Both oils were rich in sesquiterpene hydrocarbons as well as their oxygenated derivatives. Essential oils were shown to have broad spectrum antibacterial activity with MIC values that ranged from 62.5 mug/mL to 400 mug/mL, and IC(50) values from 36.93 mug/mL to 374.72 mug/mL. The oils were also shown to have moderate antifungal activity to Candida albicans growth as well as inhibition of spore germination of Magnaporthe oryzae. Two essential oils were assessed by 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging, beta-carotene bleaching and ferrozine-ferrous ions assays, respectively, to show moderate antioxidant activity. Results suggest that the isolated essential oils could be used for future development of antimicrobial and antioxidant agents.
Revision of the absolute configuration at C(23) of lanostanoids and isolation of secondary metabolites from formosan soft coral Nephthea erecta.[Pubmed:19180458]
Chem Biodivers. 2009 Jan;6(1):86-95.
Three new oxygenated ergostanoids, 1-3, one known ergostanoid, 4, one new trinoreudesmadienone, 5, one new calamenene type sesquiterpene, 6, and one known aristolane-type sesquiterpene, (-)-aristolone (7), have been isolated from the AcOEt extract of the soft coral Nephthea erecta. The structures of these compounds were determined by extensive spectroscopic and X-ray crystallographic analysis, as well as Mosher's method. We revised the absolute configuration at C(23) in the side chain of some lanostanoids as a result of the Mosher's products of 1 (i.e., 1a and 1b). The cytotoxicities against selected cancer cells and the anti-inflammatory effects of these tested metabolites 1-7 were determined in vitro.
Sesquiterpenes from the sponge Axinyssa isabela.[Pubmed:19007287]
J Nat Prod. 2008 Dec;71(12):2004-10.
Further research on the constituents of the sponge Axinyssa isabela collected in the Gulf of California has led to the isolation of nine new sesquiterpenes, the eudesmanes axinisothiocyanates M and N (1, 2), the bisabolane axinythiocyanate A (3), and the aristolane derivatives axinysones A-E (4-8) and axinynitrile A (9), together with four known sesquiterpenoids (10-13). The structures of the new metabolites have been established by spectroscopic techniques.The absolute configuration of axinysones A (4) and B (5) has been assigned after esterification with (R)- and (S)-MPA acids. In addition, the unusual nitrile-containing sesquiterpene 9 has been synthesized from (+)-aristolone (14). The cytotoxic activity of the compounds isolated has been tested against three human tumor cell lines.


