SulbactamCAS# 68373-14-8 |
- Hyoscyamine
Catalog No.:BCN1946
CAS No.:101-31-5
- Neostigmine Bromide
Catalog No.:BCC4563
CAS No.:114-80-7
- Tropicamide
Catalog No.:BCC4574
CAS No.:1508-75-4
- Otilonium Bromide
Catalog No.:BCC4573
CAS No.:26095-59-0
- Flavoxate hydrochloride
Catalog No.:BCC5208
CAS No.:3717-88-2
- Homatropine Methylbromide
Catalog No.:BCC4571
CAS No.:80-49-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 68373-14-8 | SDF | Download SDF |
| PubChem ID | 130313 | Appearance | Powder |
| Formula | C8H11NO5S | M.Wt | 233.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CP45899 | ||
| Solubility | DMSO : ≥ 100 mg/mL (428.74 mM) H2O : 20 mg/mL (85.75 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
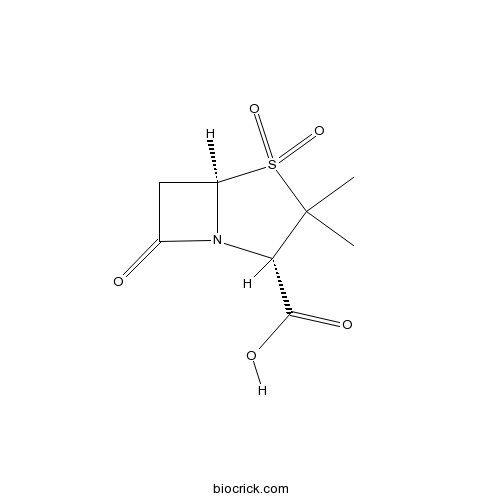
| Chemical Name | (2S,5R)-3,3-dimethyl-4,4,7-trioxo-4$l^{6}-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid | ||
| SMILES | CC1(C(N2C(S1(=O)=O)CC2=O)C(=O)O)C | ||
| Standard InChIKey | FKENQMMABCRJMK-RITPCOANSA-N | ||
| Standard InChI | InChI=1S/C8H11NO5S/c1-8(2)6(7(11)12)9-4(10)3-5(9)15(8,13)14/h5-6H,3H2,1-2H3,(H,11,12)/t5-,6+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sulbactam(Betamaze) is an irreversible β-lactamase inhibitor.
Target: β-lactamase; Antibacterial
Sulbactam is a mechanism-based inhibitor of beta-lactamase enzymes used in clinical practice. sulbactam was the antimicrobial agent responsible for the killing of these organisms [1]. sulbactam may prove effective for non-life-threatening A. baumannii infections. Its role in the treatment of severe infections is unknown. However, the current formulation of sulbactam alone may allow its use at higher doses and provide new potential synergic combinations, particularly for those infections by A. baumannii resistant to imipenem [2]. References: | |||||

Sulbactam Dilution Calculator

Sulbactam Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2874 mL | 21.4371 mL | 42.8743 mL | 85.7486 mL | 107.1857 mL |
| 5 mM | 0.8575 mL | 4.2874 mL | 8.5749 mL | 17.1497 mL | 21.4371 mL |
| 10 mM | 0.4287 mL | 2.1437 mL | 4.2874 mL | 8.5749 mL | 10.7186 mL |
| 50 mM | 0.0857 mL | 0.4287 mL | 0.8575 mL | 1.715 mL | 2.1437 mL |
| 100 mM | 0.0429 mL | 0.2144 mL | 0.4287 mL | 0.8575 mL | 1.0719 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sulbactam
- Micheliolide
Catalog No.:BCN8257
CAS No.:68370-47-8
- 7-Methoxy-1-naphthaleneacetic acid
Catalog No.:BCN2243
CAS No.:6836-22-2
- 7-Methoxy-1-naphthaleneacetic acid ethyl ester
Catalog No.:BCN1379
CAS No.:6836-21-1
- 7-Methoxy-1-tetralone
Catalog No.:BCN2241
CAS No.:6836-19-7
- Deltaline
Catalog No.:BCN5404
CAS No.:6836-11-9
- 23,24-dihydroisocucurbitacin B
Catalog No.:BCN7876
CAS No.:68354-21-2
- 1-Methyl-2-nonylquinolin-4(1H)-one
Catalog No.:BCN6585
CAS No.:68353-24-2
- Nonactin
Catalog No.:BCC7388
CAS No.:6833-84-7
- Imbricatolic acid
Catalog No.:BCN4241
CAS No.:6832-60-6
- Aristolone
Catalog No.:BCN4039
CAS No.:6831-17-0
- Amlexanox
Catalog No.:BCC8817
CAS No.:68302-57-8
- Amlexanox ethyl ester
Catalog No.:BCC8818
CAS No.:68301-99-5
- JNJ 5207787
Catalog No.:BCC6100
CAS No.:683746-68-1
- Curassavine
Catalog No.:BCN1964
CAS No.:68385-70-6
- Kuwanon E
Catalog No.:BCN3287
CAS No.:68401-05-8
- Ginsenoside Rb3
Catalog No.:BCN1065
CAS No.:68406-26-8
- 20-O-Glucoginsenoside Rf
Catalog No.:BCN8220
CAS No.:68406-27-9
- 6',7'-Dihydroxybergamottin acetonide
Catalog No.:BCN4242
CAS No.:684217-08-1
- Timosaponin AI
Catalog No.:BCN7819
CAS No.:68422-00-4
- PPDA
Catalog No.:BCC5918
CAS No.:684283-16-7
- Isowighteone
Catalog No.:BCN4243
CAS No.:68436-47-5
- 4-(p-Biphenylyl)-3-hydroxybutyric acid
Catalog No.:BCN2240
CAS No.:6845-17-6
- Heliovicine
Catalog No.:BCN2047
CAS No.:68473-85-8
- Coromandaline
Catalog No.:BCN2044
CAS No.:68473-86-9
Spectroscopy analysis and molecular dynamics studies on the binding of penicillin V and sulbactam to beta-lactamase II from Bacillus cereus.[Pubmed:28219797]
J Pharm Biomed Anal. 2017 May 10;138:206-214.
The molecular recognition and interaction of beta-lactamase II from Bacillus cereus (Bc II) with penicillin V (PV) and Sulbactam (Sul) especially conformational changes of Bc II in the binding process were studied through spectroscopy analysis in combination with molecular dynamics (MD) simulation. The results show that in the binding process, a new coordination bond is observed between the Zn2 of Bc II and the carboxyl-O of PV or Sul by replacing His204. Electrostatic interaction between Zn2 and the ligand provide main driving force for the binding affinity. Compared with apo Bc II, there are mainly four loops showing significant conformational changes in ligand-bound Bc II. A weak conformational transformation from beta-sheets to random coils is observed in the loop2 of ligand-bound Bc II. The conformational transformation may depend on the functional group and binding pose of the ligand, giving the binding pocket greater flexibility and accordingly allowing for an induced fit of the enzyme-ligand binding site around the newly introduced ligand. The change in the loop2 of ligand-bound Bc II may lead to the opening of the binding pocket of Bc II. Therefore, loop2 can be considered a gate for control of ligand access in Bc II, hence its dynamic response should be considered in new drug design and development.
Antimicrobial activity of tigecycline and cefoperazone/sulbactam tested against 18,386 Gram-negative organisms from Europe and the Asia-Pacific region (2013-2014).[Pubmed:28341098]
Diagn Microbiol Infect Dis. 2017 Jun;88(2):177-183.
A total of 18,386 organisms, including 13,224 Enterobacteriaceae, 3536 Pseudomonas aeruginosa, 1254 Acinetobacter spp., and 372Stenotrophomonas maltophilia were collected from Western Europe (WEU; n=10,021), Eastern Europe (EEU; n=4957), and the Asia-Pacific region (APAC; n=3408 [1052 from China]) in 2013-2014 as part of the SENTRY Antimicrobial Surveillance Program and tested by a reference broth microdilution method for susceptibility against tigecycline, cefoperazone/Sulbactam, and comparator agents. Overall, 95.3% of Enterobacteriaceae were susceptible (Sulbactam inhibited 94.6/83.5/91.5% of Enterobacteriaceae at
An Evaluation of Selected Indications and Appropriateness of Ampicillin/Sulbactam, an Unrestricted Antimicrobial, at a Single Center.[Pubmed:28250702]
P T. 2017 Mar;42(3):189-194.
BACKGROUND: With antimicrobial resistance on the rise and few new agents in development, it is important to exercise prudent judgment when utilizing antimicrobials. The Antimicrobial Stewardship Program (ASP) is responsible for facilitating the appropriate use of antimicrobials at the institution. Restricted antimicrobials and select additional antimicrobials are monitored by the ASP team to determine if the indications chosen by the ordering prescribers correspond to and are appropriate for the patients' infections. The purpose of this study was to review ampicillin/Sulbactam, an unrestricted antimicrobial, due to its declining effectiveness against Escherichia coli. METHODS: A retrospective chart review was conducted with adult inpatients receiving ampicillin/Sulbactam. One hundred consecutive orders for ampicillin/Sulbactam were reviewed. RESULTS: The greatest number of orders for ampicillin/Sulbactam came from the Emergency Trauma, Medicine, and Surgery services. The indications selected by the ordering providers were: skin and soft tissue infection (33 orders), community-acquired respiratory infection (22 orders), other (14 orders), intra-abdominal infection due to susceptible organism (13 orders), urinary tract infection (nine orders), head/neck infection (five orders), infection due to human or animal bite (three orders), and diabetic foot infection (one order). CONCLUSIONS: The correct indication was selected in 78% of orders that were reviewed for correctness. Empiric ampicillin/Sulbactam was appropriate in 51% of orders, with the majority of inappropriate empiric usage being intra-abdominal and urinary tract infections.
Safety and efficacy of a novel drug elores (ceftriaxone+sulbactam+disodium edetate) in the management of multi-drug resistant bacterial infections in tertiary care centers: a post-marketing surveillance study.[Pubmed:28376315]
Braz J Infect Dis. 2017 Jul - Aug;21(4):408-417.
OBJECTIVE: In India, Elores (CSE-1034: ceftriaxone+Sulbactam+disodium edetate) was approved as a broad spectrum antibiotic in year 2011 and is used for management of Extended Spectrum Beta Lactamases/Metallo Beta lactamases infections in tertiary care centers. The objective of this study was to investigate the efficacy of this drug in patients with Extended Spectrum Beta Lactamases/Metallo Beta lactamases infections and identify the incidence of adverse events in real clinical settings. METHODS: This Post Marketing Surveillance study was conducted at 17 centers across India and included 2500 patients of all age groups suffering from various bacterial infections and treated with Elores (CSE1034). Information regarding demographic, clinical and microbiological parameters, dosage and treatment duration, efficacy and adverse events (AEs) associated with the treatment were recorded. RESULTS: A total of 2500 patients were included in the study and efficacy was evaluated in 2487 patients. In total, 409 AEs were reported in 211 (8.4%) patients. The major AEs reported were vomiting (3.0%), pain at injection site (2.5%), nausea (2.3%), redness at site (1.96%), thrombophlebitis (1.4%). Of total reported AEs, 40 (5.3%) AEs were reported in pediatric, 310 (20.6%) in adult, and 59 (23.6%) in geriatric group. No AE belonging to grade IV or V was reported in any patient. In terms of efficacy, 1977 (79.4%) patients were cured, 501 (20.1%) patients showed clinical improvement and 5 (0.2%) patients were complete failure. The treatment duration varied from 5 to 7 days in different patients depending on the infection type. CONCLUSION: In this post-marketing surveillance study, CSE-1034 was found to be an effective and safe option against Pip tazo and meropenem in management of patients with multi-drug resistant (MDR) bacterial infections under routine ward settings.


