Zonisamide sodiumCAS# 68291-98-5 |
- Mibefradil
Catalog No.:BCC1748
CAS No.:116644-53-2
- Mibefradil dihydrochloride
Catalog No.:BCC1749
CAS No.:116666-63-8
- Cilnidipine
Catalog No.:BCC1083
CAS No.:132203-70-4
- NNC 55-0396
Catalog No.:BCC1803
CAS No.:357400-13-6
- NP118809
Catalog No.:BCC1807
CAS No.:41332-24-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 68291-98-5 | SDF | Download SDF |
| PubChem ID | 4099656 | Appearance | Powder |
| Formula | C8H7N2NaO3S | M.Wt | 234.21 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
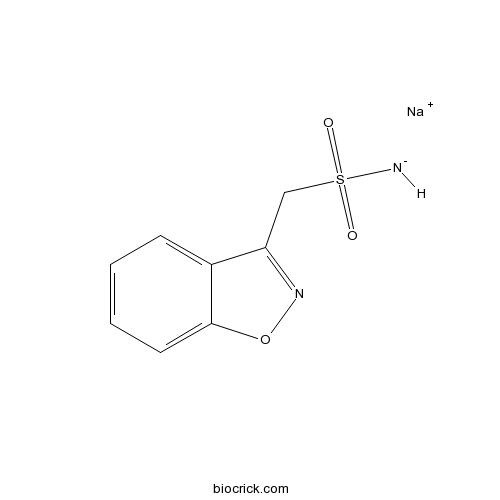
| Chemical Name | sodium;1,2-benzoxazol-3-ylmethylsulfonylazanide | ||
| SMILES | C1=CC=C2C(=C1)C(=NO2)CS(=O)(=O)[NH-].[Na+] | ||
| Standard InChIKey | ZVBIRPKGWOVBLG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H7N2O3S.Na/c9-14(11,12)5-7-6-3-1-2-4-8(6)13-10-7;/h1-4H,5H2,(H-,9,11,12);/q-1;+1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Zonisamide sodium Dilution Calculator

Zonisamide sodium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2697 mL | 21.3484 mL | 42.6967 mL | 85.3935 mL | 106.7418 mL |
| 5 mM | 0.8539 mL | 4.2697 mL | 8.5393 mL | 17.0787 mL | 21.3484 mL |
| 10 mM | 0.427 mL | 2.1348 mL | 4.2697 mL | 8.5393 mL | 10.6742 mL |
| 50 mM | 0.0854 mL | 0.427 mL | 0.8539 mL | 1.7079 mL | 2.1348 mL |
| 100 mM | 0.0427 mL | 0.2135 mL | 0.427 mL | 0.8539 mL | 1.0674 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Zonisamide sodium is a 1,2 benzisoxazole derivative and the first agent of this chemical class to be developed as an antiepileptic drug.
- Zonisamide
Catalog No.:BCC2512
CAS No.:68291-97-4
- Boc-Arg(Mts)-OH.CHA
Catalog No.:BCC3045
CAS No.:68262-71-5
- (R)-Oxiracetam
Catalog No.:BCC4169
CAS No.:68252-28-8
- 2-Hydroxy-3-(hydroxymethyl)anthraquinone
Catalog No.:BCN1380
CAS No.:68243-30-1
- 6-Prenylnaringenin
Catalog No.:BCN2999
CAS No.:68236-13-5
- 6,8-Diprenylnaringenin
Catalog No.:BCN3000
CAS No.:68236-11-3
- BRL 44408 maleate
Catalog No.:BCC6948
CAS No.:681806-46-2
- Isoliensinine
Catalog No.:BCN6331
CAS No.:6817-41-0
- Nortetraphyllicine
Catalog No.:BCN4240
CAS No.:68160-76-9
- 1-O-Acetyl britannilactone
Catalog No.:BCN2365
CAS No.:681457-46-5
- Jujuboside B1
Catalog No.:BCN3881
CAS No.:68144-21-8
- Humic acid sodium salt
Catalog No.:BCN1284
CAS No.:68131-04-4
- Planinin
Catalog No.:BCC8981
CAS No.:68296-27-5
- Amlexanox ethyl ester
Catalog No.:BCC8818
CAS No.:68301-99-5
- Amlexanox
Catalog No.:BCC8817
CAS No.:68302-57-8
- Aristolone
Catalog No.:BCN4039
CAS No.:6831-17-0
- Imbricatolic acid
Catalog No.:BCN4241
CAS No.:6832-60-6
- Nonactin
Catalog No.:BCC7388
CAS No.:6833-84-7
- 1-Methyl-2-nonylquinolin-4(1H)-one
Catalog No.:BCN6585
CAS No.:68353-24-2
- 23,24-dihydroisocucurbitacin B
Catalog No.:BCN7876
CAS No.:68354-21-2
- Deltaline
Catalog No.:BCN5404
CAS No.:6836-11-9
- 7-Methoxy-1-tetralone
Catalog No.:BCN2241
CAS No.:6836-19-7
- 7-Methoxy-1-naphthaleneacetic acid ethyl ester
Catalog No.:BCN1379
CAS No.:6836-21-1
- 7-Methoxy-1-naphthaleneacetic acid
Catalog No.:BCN2243
CAS No.:6836-22-2
Zonisamide enhances slow sodium inactivation in Myxicola.[Pubmed:2439177]
Brain Res. 1987 Jun 9;413(1):185-8.
In voltage-clamped Myxicola giant axons Zonisamide (1,2-benzisoxazole-3-methanesulfonamide) caused a hyperpolarizing shift in the steady-state fast inactivation curve and retarded recovery from fast and slow Na+ inactivation. The effects of Zonisamide on steady-state fast inactivation could be described assuming a single binding site with a dissociation constant of 12 microM. Slow inactivation was significantly more sensitive, with a Kd of 1 microM from both steady-state and kinetic data. While these results account for anticonvulsant activity, the differential sensitivity suggests Zonisamide may also be useful in studies of the slow inactive state of the Na+ channel.
Comparing Zonisamide With Sodium Valproate in the Management of Migraine Headaches: Double-Blind Randomized Clinical Trial of Efficacy and Safety.[Pubmed:28144450]
Iran Red Crescent Med J. 2016 Apr 30;18(9):e23768.
BACKGROUND: Migraine is one of the most debilitating medical conditions and has a high socioeconomic burden. As conventional therapeutic methods do not entirely alleviate the symptoms, new alternatives are being considered. OBJECTIVES: This study evaluates the efficacy and safety of zonisamide compared with sodium valproate in the management of migraine headaches. PATIENTS AND METHODS: In the current double-blind, parallel, randomized, controlled trial, 96 patients with a migraine diagnosis based on the international headache society (HIS) criteria were selected. They were divided randomly into two groups; the case group was given zonisamide, and sodium valproate was given to a control group. In addition to the side effects of the drugs, the severity, duration, and frequency of migraine attacks were evaluated at baseline and at three months. RESULTS: The 96 selected patients were divided randomly into two treatment groups (zonisamide n = 48, sodium valproate n = 48). Seven patients were excluded from analysis because of early dropout, leaving 89 (n = 45; n = 44) patients for analysis. While using zonisamide, six (13%) patients complained of fatigue, and two (4%) patients encountered noticeable appetite and weight loss. In the control group, five (11%) patients reported dizziness, and four (9%) patients faced obvious appetite and weight gain. Both drugs were considerably efficient in reducing further attacks. There was no statistically significant correlation between frequency or severity of migraine attacks and the drug used for treatment in three months of follow-up. CONCLUSIONS: Both medications are effective in reducing migraine attacks. It will be important to consider the drugs' adverse effects and availability and patients' medical and socioeconomic condition to select the appropriate treatment.
Drug interactions of zonisamide with phenytoin and sodium valproate: serum concentrations and protein binding.[Pubmed:7573757]
Brain Dev. 1995 May-Jun;17(3):182-5.
The influence of co-medication with zonisamide (ZNS) on the serum concentration and protein binding of phenytoin (PHT) and sodium valproate (VPA) was studied in 21 pediatric patients. No significant correlation between the daily ZNS dose, and total serum concentrations, free concentrations or free fractions (FF) of PHT or VPA was observed. The patient study showed that changes in the FF of PHT and VPA were correlated more closely with the serum protein and bilirubin levels than changes in the ZNS dosage. An in vitro study revealed that the addition of ZNS caused decreases in the FF of PHT and VPA. However, these decreases were within the range of measurement error and were negligible. In conclusion, no significant effect of ZNS on the serum concentration or protein binding of PHT or VPA was demonstrated.
[Valproate sodium and zonisamide associated interstitial pneumonitis in an infant].[Pubmed:17228818]
No To Hattatsu. 2007 Jan;39(1):44-8.
A six month-old female infant developed dyspnea when she was treated with valproate sodium (VPA) and zonisamide (ZNS) for epileptic spasms. Two weeks after its onset, her dyspnea was exacerbated by respiratory syncytial virus (RSV) infection. We diagnosed interstitial pneumonitis (IP) based on her chest CT and high serum concentrations of KL-6 and surfactant protein D. Her dyspnea improved with the treatment which included steroids, neutrophil elastase inhibitor and discontinuation of VPA and ZNS. An allergic reaction probably explains the IP in our patient because steroids were effective. Her IP might have been exacerbated by several factors including the use of two anticonvulsants (VPA, ZNS), RSV infection, and physiological low serum IgA level of infants. Drug-induced interstitial lung disease should be remembered as a possible complication of anticonvulsant treatment, such as VPA and ZNS.


