DeltalineCAS# 6836-11-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6836-11-9 | SDF | Download SDF |
| PubChem ID | 441728 | Appearance | White powder |
| Formula | C27H41NO8 | M.Wt | 507.62 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
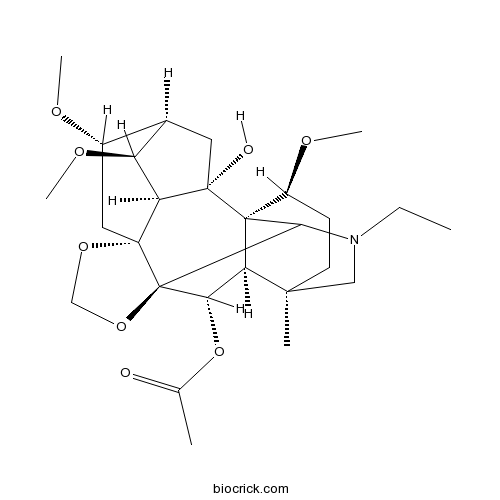
| SMILES | CCN1CC2(CCC(C34C2C(C5(C31)C6(CC(C7CC4(C6C7OC)O)OC)OCO5)OC(=O)C)OC)C | ||
| Standard InChIKey | DTTPWCNKTMQMTE-DZZCPBQSSA-N | ||
| Standard InChI | InChI=1S/C27H41NO8/c1-7-28-12-23(3)9-8-17(32-5)26-20(23)21(36-14(2)29)27(22(26)28)25(34-13-35-27)11-16(31-4)15-10-24(26,30)19(25)18(15)33-6/h15-22,30H,7-13H2,1-6H3/t15-,16+,17+,18+,19+,20-,21+,22?,23+,24+,25-,26-,27-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Deltaline, enabling the synthesis of 32 new derivatives bearing a broad spectrum of unique scaffolds. |
| Structure Identification | Chemistry. 2015 Jun 8;21(24):8946-50.Generating skeletal diversity from the c19 -diterpenoid alkaloid deltaline: a ring-distortion approach.[Pubmed: 25950550]The development of new drugs calls for large collections of diverse molecules with considerable complexity. Ring distortion of natural products provides an efficient and facile approach to access new architectures with intriguing biological activities, by harnessing their inherent complexity. Nat Prod Commun. 2012 Jun;7(6):721-4.Unusual reactions of a 7,17-seco-type C19-diterpenoid alkaloid derived from deltaline.[Pubmed: 22816291]Treatment of a 7,17-seco-type C19-diterpenoid alkaloid (3), prepared from Deltaline (8), with triethylamine in either DMF or TEG (triethylene glycol) at 120 degrees C provided two interesting compounds 6 and 7. The structures of compounds 3, 6, and 7 were established based on extensive interpretations of their 1D and 2D NMR data. Compound 6, a lycoctonine-type C19-diterpenoid alkaloid, can be transformed from alkaloid 3 via Grob fragmentation, Prins reaction, and intramolecular disproportionation. The mechanism of the formation of compound 6 was confirmed by deuteration experiments. Product 7 was formed through a pinacol-like rearrangement of alkaloid 3. |

Deltaline Dilution Calculator

Deltaline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.97 mL | 9.8499 mL | 19.6998 mL | 39.3996 mL | 49.2494 mL |
| 5 mM | 0.394 mL | 1.97 mL | 3.94 mL | 7.8799 mL | 9.8499 mL |
| 10 mM | 0.197 mL | 0.985 mL | 1.97 mL | 3.94 mL | 4.9249 mL |
| 50 mM | 0.0394 mL | 0.197 mL | 0.394 mL | 0.788 mL | 0.985 mL |
| 100 mM | 0.0197 mL | 0.0985 mL | 0.197 mL | 0.394 mL | 0.4925 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 23,24-dihydroisocucurbitacin B
Catalog No.:BCN7876
CAS No.:68354-21-2
- 1-Methyl-2-nonylquinolin-4(1H)-one
Catalog No.:BCN6585
CAS No.:68353-24-2
- Nonactin
Catalog No.:BCC7388
CAS No.:6833-84-7
- Imbricatolic acid
Catalog No.:BCN4241
CAS No.:6832-60-6
- Aristolone
Catalog No.:BCN4039
CAS No.:6831-17-0
- Amlexanox
Catalog No.:BCC8817
CAS No.:68302-57-8
- Amlexanox ethyl ester
Catalog No.:BCC8818
CAS No.:68301-99-5
- Planinin
Catalog No.:BCC8981
CAS No.:68296-27-5
- Zonisamide sodium
Catalog No.:BCC4240
CAS No.:68291-98-5
- Zonisamide
Catalog No.:BCC2512
CAS No.:68291-97-4
- Boc-Arg(Mts)-OH.CHA
Catalog No.:BCC3045
CAS No.:68262-71-5
- (R)-Oxiracetam
Catalog No.:BCC4169
CAS No.:68252-28-8
- 7-Methoxy-1-tetralone
Catalog No.:BCN2241
CAS No.:6836-19-7
- 7-Methoxy-1-naphthaleneacetic acid ethyl ester
Catalog No.:BCN1379
CAS No.:6836-21-1
- 7-Methoxy-1-naphthaleneacetic acid
Catalog No.:BCN2243
CAS No.:6836-22-2
- Micheliolide
Catalog No.:BCN8257
CAS No.:68370-47-8
- Sulbactam
Catalog No.:BCC4941
CAS No.:68373-14-8
- JNJ 5207787
Catalog No.:BCC6100
CAS No.:683746-68-1
- Curassavine
Catalog No.:BCN1964
CAS No.:68385-70-6
- Kuwanon E
Catalog No.:BCN3287
CAS No.:68401-05-8
- Ginsenoside Rb3
Catalog No.:BCN1065
CAS No.:68406-26-8
- 20-O-Glucoginsenoside Rf
Catalog No.:BCN8220
CAS No.:68406-27-9
- 6',7'-Dihydroxybergamottin acetonide
Catalog No.:BCN4242
CAS No.:684217-08-1
- Timosaponin AI
Catalog No.:BCN7819
CAS No.:68422-00-4
Unusual reactions of a 7,17-seco-type C19-diterpenoid alkaloid derived from deltaline.[Pubmed:22816291]
Nat Prod Commun. 2012 Jun;7(6):721-4.
Treatment of a 7,17-seco-type C19-diterpenoid alkaloid (3), prepared from Deltaline (8), with triethylamine in either DMF or TEG (triethylene glycol) at 120 degrees C provided two interesting compounds 6 and 7. The structures of compounds 3, 6, and 7 were established based on extensive interpretations of their 1D and 2D NMR data. Compound 6, a lycoctonine-type C19-diterpenoid alkaloid, can be transformed from alkaloid 3 via Grob fragmentation, Prins reaction, and intramolecular disproportionation. The mechanism of the formation of compound 6 was confirmed by deuteration experiments. Product 7 was formed through a pinacol-like rearrangement of alkaloid 3.


