WIN 18446ALDH1a2 inhibitor CAS# 1477-57-2 |
- MK-4827
Catalog No.:BCC1761
CAS No.:1038915-60-4
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- PJ34
Catalog No.:BCC1865
CAS No.:344458-19-1
- ABT-888 (Veliparib)
Catalog No.:BCC1267
CAS No.:912444-00-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1477-57-2 | SDF | Download SDF |
| PubChem ID | 15134 | Appearance | Powder |
| Formula | C12H20Cl4N2O2 | M.Wt | 366.11 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 20 mM in ethanol | ||
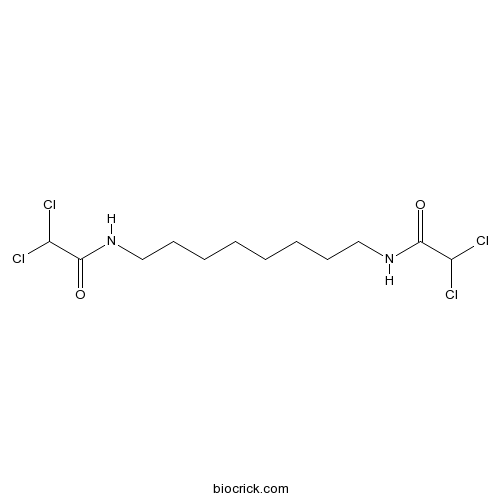
| Chemical Name | 2,2-dichloro-N-[8-[(2,2-dichloroacetyl)amino]octyl]acetamide | ||
| SMILES | C(CCCCNC(=O)C(Cl)Cl)CCCNC(=O)C(Cl)Cl | ||
| Standard InChIKey | FAOMZVDZARKPFJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H20Cl4N2O2/c13-9(14)11(19)17-7-5-3-1-2-4-6-8-18-12(20)10(15)16/h9-10H,1-8H2,(H,17,19)(H,18,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of aldehyde dehydrogenase 1a2 (ALDH1a2) (IC50 = 0.3 μM). Inhibits the biosynthesis of retinoic acid from retinol in neonatal and adult murine testis. |

WIN 18446 Dilution Calculator

WIN 18446 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7314 mL | 13.6571 mL | 27.3142 mL | 54.6284 mL | 68.2855 mL |
| 5 mM | 0.5463 mL | 2.7314 mL | 5.4628 mL | 10.9257 mL | 13.6571 mL |
| 10 mM | 0.2731 mL | 1.3657 mL | 2.7314 mL | 5.4628 mL | 6.8285 mL |
| 50 mM | 0.0546 mL | 0.2731 mL | 0.5463 mL | 1.0926 mL | 1.3657 mL |
| 100 mM | 0.0273 mL | 0.1366 mL | 0.2731 mL | 0.5463 mL | 0.6829 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-Amino-4-methylbenzothiazole
Catalog No.:BCC8533
CAS No.:1477-42-5
- ZM 226600
Catalog No.:BCC6831
CAS No.:147695-92-9
- Magnolianin
Catalog No.:BCN3985
CAS No.:147663-91-0
- Calcipotriol monohydrate
Catalog No.:BCC1445
CAS No.:147657-22-5
- 5-Chloro-4-methoxy-2-oxo-1,2-dihydropyridine-3-carbonitrile
Catalog No.:BCC8744
CAS No.:147619-40-7
- Novobiocin Sodium
Catalog No.:BCC4812
CAS No.:1476-53-5
- ID-8
Catalog No.:BCC4787
CAS No.:147591-46-6
- DiMNF
Catalog No.:BCC3900
CAS No.:14756-24-2
- Racemodine
Catalog No.:BCN2023
CAS No.:147554-28-7
- trans-3-Hydroxycinnamic acid
Catalog No.:BCN5029
CAS No.:14755-02-3
- Bosentan
Catalog No.:BCC4640
CAS No.:147536-97-8
- Pitavastatin Calcium
Catalog No.:BCC3842
CAS No.:147526-32-7
- 8-(3-Chlorostyryl)caffeine
Catalog No.:BCC7640
CAS No.:147700-11-6
- 6-O-(3'',4''-Dimethoxycinnamoyl)catalpol
Catalog No.:BCN1655
CAS No.:147714-71-4
- 3,4-Dimethoxybenzenepropanamine
Catalog No.:BCN1785
CAS No.:14773-42-3
- Fmoc-O-Phospho-Tyr-OH
Catalog No.:BCC3563
CAS No.:147762-53-6
- Repaglinide ethyl ester
Catalog No.:BCC9135
CAS No.:147770-06-7
- L-161,982
Catalog No.:BCC7393
CAS No.:147776-06-5
- DCG IV
Catalog No.:BCC5691
CAS No.:147782-19-2
- ω-Conotoxin MVIIC
Catalog No.:BCC5699
CAS No.:147794-23-8
- Santacruzamate A (CAY10683)
Catalog No.:BCC5488
CAS No.:1477949-42-0
- Cefcapene pivoxil hydrochloride
Catalog No.:BCC8906
CAS No.:147816-24-8
- Siramesine
Catalog No.:BCC4304
CAS No.:147817-50-3
- Niazimicin
Catalog No.:BCN7641
CAS No.:147821-49-6
Testicular toxicity of WIN 18446 in the laboratory mouse.[Pubmed:8563190]
Reprod Toxicol. 1995 Sep-Oct;9(5):475-81.
The effect of oral administration of N,N'=bis (dichloroacetyl)-1, 8-octamethylenediamine (WIN 18446) (200 mg/kg body weight/day, up to 30 days) on the testis of the Parkes (P) strain laboratory mouse was studied. The drug caused reduction in testicular weight and severe atrophic changes in the seminiferous tubules. A duration-dependent effect of the drug was observed on the germ cells. The drug had its initial impact on spermatids followed by spermatocytes, ultimately culminating in the Sertoli cell-spermatogonia syndrome. The drug-induced changes included exfoliation of germ cells, formation of multinucleated giant cells, and vacuolization of cytoplasm and displacement (towards the lumina of the tubules) of Sertoli cell nuclei. Even 75 days after drug withdrawal, testicular weight remained depressed and in 15 to 20% of the tubules there was incomplete recovery of spermatogenesis. Our data indicate that WIN 18446 induces sustained impairment of spermatogenesis by a direct action on spermatogonia or indirectly by affecting the integrity of the Sertoli cells. The Leydig cells remained unaffected in WIN 18446-treated mice.
Short-term effects of N'N-bis(dichloroacetyl)-1,8-octamethylenediamine (WIN 18446) on the testes, selected sperm parameters and fertility of male CBA mice.[Pubmed:14599310]
Lab Anim. 2003 Oct;37(4):363-73.
N'N-bis(dichloroacetyl)-1,8-octamethylenediamine (WIN 18446), the most potent of the diamines and one of the least amoebicidal agents, was shown to exert a specific effect on the testes of CBA mice, while the Leydig cells were unaffected. Spermatogenesis was severely affected after a 42-day treatment period with 125 mg WIN 18446/kg body weight. Large multinucleated cells, vacuolization and the absence of sperm within the testes were evident in most seminiferous tubules. After 15 days of withdrawal of WIN 18446, there was a slight recovery of spermatogenesis and after withdrawal of 42 days a marked recovery of spermatogenesis. The normality or abnormality of this spermatogenic cycle could be evaluated using the semi-quantitative Stages program. There was a significant decrease in the diameters of seminiferous tubules of WIN 18446 treated mice, however an almost complete recovery was evident after 42 days of withdrawal of WIN 18446. A significant decrease in sperm concentration and sperm morphology was observed for the WIN 18446 treated mice. Various sperm motion parameters were assessed for the different treatment groups and compared to the control group. The female and male fertility indices were assessed and compared for the different treatment groups. Complete recovery of the above-mentioned parameters was evident after 42 days of withdrawal from WIN 18446, and this confirms its potential as a possible contraceptive for animal populations.
Inhibition of retinoic acid biosynthesis by the bisdichloroacetyldiamine WIN 18,446 markedly suppresses spermatogenesis and alters retinoid metabolism in mice.[Pubmed:24711451]
J Biol Chem. 2014 May 23;289(21):15104-17.
Knowledge of the regulation of testicular retinoic acid synthesis is crucial for understanding its role in spermatogenesis. Bisdichloroacetyldiamines strongly inhibit spermatogenesis. We reported previously that one of these compounds, WIN 18,446, potently inhibited spermatogenesis in rabbits by inhibiting retinoic acid synthesis. To understand how WIN 18,446 inhibits retinoic acid synthesis, we characterized its effects on human retinal dehydrogenase ALDH1A2 in vitro as well as its effects on retinoid metabolism in vivo using mice. WIN 18,446 strongly and irreversibly inhibited ALDH1A2 in vitro. In vivo, WIN 18,446 treatment completely abolished spermatogenesis after 4 weeks of treatment and modestly reduced adiposity in mice fed a chow diet. Effects of WIN 18,446 on retinoid concentrations were tissue-dependent. Although lung and liver retinyl ester concentrations were lower in WIN 18,446-treated animals, adipose retinyl ester levels were increased following the treatment. Interestingly, animals treated with WIN 18,446 had significantly higher circulating retinol concentrations compared with control mice. The effect on spermatogenesis by WIN 18,446 was not prevented by simultaneous treatment with retinoic acid, whereas effects on other tissues were partially or completely reversed. Cessation of WIN 18,446 treatment for 4 weeks reversed most retinoid-related phenotypes except for inhibition of spermatogenesis. Our data suggest that WIN 18,446 may be a useful model of systemic acquired retinoic acid deficiency. Given the effects observed in our study, inhibition of retinoic acid biosynthesis may have relevance for the treatment of obesity and in the development of novel male contraceptives.
Suppression of spermatogenesis by bisdichloroacetyldiamines is mediated by inhibition of testicular retinoic acid biosynthesis.[Pubmed:20705791]
J Androl. 2011 Jan-Feb;32(1):111-9.
The bisdichloroacetyldiamine WIN 18,446 reversibly inhibits spermatogenesis in many species, including humans; however, the mechanism by which WIN 18,446 functions is unknown. As retinoic acid is essential for spermatogenesis, we hypothesized that WIN 18,446 might inhibit retinoic acid biosynthesis from retinol (vitamin A) within the testes by inhibiting the enzyme aldehyde dehydrogenase 1a2 (ALDH1a2). We studied the effect of WIN 18,446 on ALDH1a2 enzyme activity in vitro, and on spermatogenesis and fertility in vivo, in mature male rabbits for 16 weeks. WIN 18,446 markedly inhibited ALDH1a2 enzyme activity in vitro with an IC(50) of 0.3 muM. In vivo, the oral administration of 200 mg/kg WIN 18,446 to male rabbits for 16 weeks significantly reduced intratesticular concentrations of retinoic acid, severely impaired spermatogenesis, and caused infertility. Reduced concentrations of intratesticular retinoic acid were apparent after only 4 weeks of treatment and preceded the decrease in sperm counts and the loss of mature germ cells in tissue samples. Sperm counts and fertility recovered after treatment was discontinued. These findings demonstrate that bisdichloroacetyldiamines such as WIN 18,446 reversibly suppress spermatogenesis via inhibition of testicular retinoic acid biosynthesis by ALDH1a2. These findings suggest that ALDH1a2 is a promising target for the development of a reversible, nonhormonal male contraceptive.
Suppression of Stra8 expression in the mouse gonad by WIN 18,446.[Pubmed:21209416]
Biol Reprod. 2011 May;84(5):957-65.
Bis-(dichloroacetyl)-diamines (BDADs) are compounds that inhibit spermatogenesis and function as male contraceptives in many species; however, their mechanism of action has yet to be fully investigated. It has been proposed that BDADs may function via inhibition of testicular retinoic acid (RA) biosynthesis. We employed an organ culture technique and the expression of a marker for RA activity, Stra8 (stimulated by retinoic acid gene 8), to investigate if the BDAD WIN 18,446 inhibited the biosynthesis of RA from retinol (ROL) in neonatal and adult murine testis and in the embryonic murine gonad. After culturing either whole testes or germ cells isolated from mice at 2 days postpartum (dpp) with WIN 18,446 or with WIN 18,446 plus ROL, Stra8 expression was suppressed, demonstrating that WIN 18,446 inhibited the conversion of ROL to RA in both systems. We also utilized a transgenic mouse containing an RA-responsive LacZ reporter gene to demonstrate limited RA induction of LacZ expression in 2-dpp testes cultured with WIN 18,446 plus ROL. The expression of Stra8 was downregulated in adult mouse testis tubules cultured with WIN 18,446 when compared to tubules cultured with the vehicle control. WIN 18,446 also inhibited the conversion of ROL to RA in embryonic ovaries and testes cultured for 48 h. These murine results provide critical insights regarding how the BDADs can inhibit spermatogenesis by blocking the ability of vitamin A to drive germ cell development. In addition, these techniques will be useful for screening novel inhibitors of RA biosynthesis as potential male contraceptives.


