NiazimicinCAS# 147821-49-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 147821-49-6 | SDF | Download SDF |
| PubChem ID | 10247749 | Appearance | Oil |
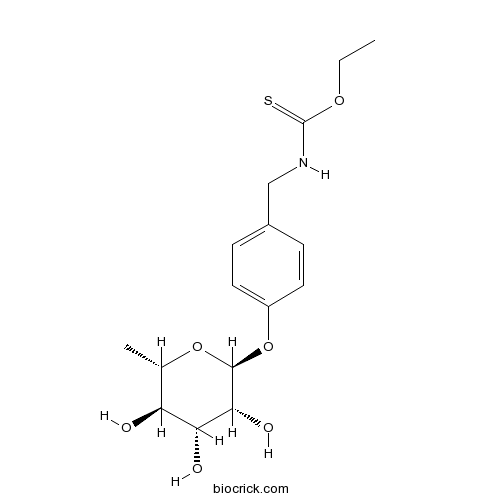
| Formula | C16H23NO6S | M.Wt | 357.42 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | O-ethyl N-[[4-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyphenyl]methyl]carbamothioate | ||
| SMILES | CCOC(=S)NCC1=CC=C(C=C1)OC2C(C(C(C(O2)C)O)O)O | ||
| Standard InChIKey | JOSHUAQJVMGTGS-NBUQLFNLSA-N | ||
| Standard InChI | InChI=1S/C16H23NO6S/c1-3-21-16(24)17-8-10-4-6-11(7-5-10)23-15-14(20)13(19)12(18)9(2)22-15/h4-7,9,12-15,18-20H,3,8H2,1-2H3,(H,17,24)/t9-,12-,13+,14+,15-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Niazimicin has potent antitumor promoting activity in the two-stage carcinogenesis in mouse skin using 7,12-dimethylbenz(a)anthracene (DMBA) as initiator and TPA as tumor promoter, it is proposed to be a potent chemo-preventive agent. 2. Niazimicin shows antimicrobial activity. 3. Niazimicin has hypotensive and spasmolytic activities. 4. Niazimicin are NF-B and PI3K inhibitors. |
| Targets | PI3K | NF-kB | AChR | Antifection |

Niazimicin Dilution Calculator

Niazimicin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7978 mL | 13.9891 mL | 27.9783 mL | 55.9566 mL | 69.9457 mL |
| 5 mM | 0.5596 mL | 2.7978 mL | 5.5957 mL | 11.1913 mL | 13.9891 mL |
| 10 mM | 0.2798 mL | 1.3989 mL | 2.7978 mL | 5.5957 mL | 6.9946 mL |
| 50 mM | 0.056 mL | 0.2798 mL | 0.5596 mL | 1.1191 mL | 1.3989 mL |
| 100 mM | 0.028 mL | 0.1399 mL | 0.2798 mL | 0.5596 mL | 0.6995 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Siramesine
Catalog No.:BCC4304
CAS No.:147817-50-3
- Cefcapene pivoxil hydrochloride
Catalog No.:BCC8906
CAS No.:147816-24-8
- Santacruzamate A (CAY10683)
Catalog No.:BCC5488
CAS No.:1477949-42-0
- ω-Conotoxin MVIIC
Catalog No.:BCC5699
CAS No.:147794-23-8
- DCG IV
Catalog No.:BCC5691
CAS No.:147782-19-2
- L-161,982
Catalog No.:BCC7393
CAS No.:147776-06-5
- Repaglinide ethyl ester
Catalog No.:BCC9135
CAS No.:147770-06-7
- Fmoc-O-Phospho-Tyr-OH
Catalog No.:BCC3563
CAS No.:147762-53-6
- 3,4-Dimethoxybenzenepropanamine
Catalog No.:BCN1785
CAS No.:14773-42-3
- 6-O-(3'',4''-Dimethoxycinnamoyl)catalpol
Catalog No.:BCN1655
CAS No.:147714-71-4
- 8-(3-Chlorostyryl)caffeine
Catalog No.:BCC7640
CAS No.:147700-11-6
- WIN 18446
Catalog No.:BCC6273
CAS No.:1477-57-2
- Niazinin
Catalog No.:BCN7623
CAS No.:147821-57-6
- Filic-3-en-25-al
Catalog No.:BCN6445
CAS No.:147850-78-0
- CA-074 Me
Catalog No.:BCC3649
CAS No.:147859-80-1
- Isokadsurenin D
Catalog No.:BCN6615
CAS No.:147976-35-0
- Dinitolmide
Catalog No.:BCC8945
CAS No.:148-01-6
- Beta-Tocopherol
Catalog No.:BCN6683
CAS No.:148-03-8
- Pilocarpin Nitrate
Catalog No.:BCC8234
CAS No.:148-72-1
- Thiabendazole
Catalog No.:BCC3868
CAS No.:148-79-8
- Melphalan
Catalog No.:BCC2403
CAS No.:148-82-3
- Doripenem
Catalog No.:BCC4094
CAS No.:148016-81-3
- 1-(3-(1-Hydroxy-3-methylbutyl)-4-methoxyphenyl)ethan-1-one
Catalog No.:BCN7493
CAS No.:148044-44-4
- 25-Hydroxycycloart-23-en-3-one
Catalog No.:BCN1657
CAS No.:148044-47-7
Synergistic antimicrobial efficacy of mesoporous ZnO loaded with 4-(alpha-L-rhamnosyloxy)-benzyl isothiocyanate isolated from the Moringa oleifera seed.[Pubmed:25742976]
J Gen Appl Microbiol. 2014;60(6):251-5.
The antimicrobial activities of isolated compounds from seed extracts of Moringa oleifera and synergistic antimicrobial efficacy through hybridized complex of organic-inorganic composite materials were studied. The two main components of the Moringa oleifera seed were isolated and determined to be Niazimicin and 4-(alpha-L-rhamnosyloxy)-benzyl isothiocyanate (RBI). The antimicrobial activity of the separated compounds of the Moringa oleifera seed were tested in vitro against 3 bacterial species and 2 fungal species by the paper disc diffusion assay and broth dilution methods. Both compounds showed antimicrobial activity against tested species and RBI was more effective than Niazimicin. The MIC of RBI on S. aureus, E. coli, P. aeruginosa, C. albicans, and A. niger was 0.005%, 0.1%, 0.5%, 0.5%, and 0.5%, respectively, while the MIC of Niazimicin on S. aureus was 0.1%. Next, we investigated the combined antimicrobial action of mesoporous ZnO and RBI by incorporating the compound within the pore of mesoporous ZnO. The MIC of mesoporous ZnO with RBI on S. aureus, E. coli, P. aeruginosa, C. albicans, and A. niger was 0.001%, 0.01%, 0.5%, 0.1%, and 0.1%, respectively. A synergistic effect of RBI with mesoporous ZnO was shown. From these results, the mesoporous ZnO could act as a reservoir for RBI and mesoporous ZnO with RBI could be used for cosmetic preservatives.
Review: an exposition of medicinal preponderance of Moringa oleifera (Lank.).[Pubmed:24577932]
Pak J Pharm Sci. 2014 Mar;27(2):397-403.
Medicinal plants are believed to be a precious natural reservoir as they are assumed to have paranormal effects for the mankind. Moringa oleifera grows throughout most of the tropics and has numerous industrial and medicinal uses. This review acquaints with the consequence of fera (Moringaceae), a fast growing medicinal plant wide spread in tropical regions with height ranging from 5-10m. It has an enormous nutritional worth due to existence of vitamins and proteins. It is subsisted with many constituents. Its oil consists of oleic, tocopherols, stearic, palmitic, behenic and arachidic acid. Flavanoids and phenolics such as gallic acid, chlorogenic acid, ferulic acid, kaempferol, ellagic acid, quercetin and vanillin are present by means of leaf extract, being richest in phenolics and subsequent fruit and seed extract respectively, that are accountable for antioxidant activity of plant. Seeds have been pragmatic with active components as novel O-ethyl-4- (alpha -L-rhamnosyloxy) benzyl carbamate together with seven known compounds, 4 (alpha -L-rhamnosyloxy)-benzyl isothiocyanate, Niazimicin, niazirin, beta-sitosterol, glycerol-1- (9 -octadecanoate), 3 -O- 6 -O-oleoyl- beta -D-glucopyranosyl-b-sitosterol, and beta - sitosterol- 3-X-O -beta -D-glucopyranoside , that have been discerned to inhibit EBV-EA (Epstein- Barr virus-early antigen), that is persuaded by the cancer promoter. M. oleifera leaves, gums, roots, flowers as well as kernels have been unanimously utilized for managing tissue tenderness, cardiovascular and liver maladies, normalize blood glucose and cholesterol. It has also profound antimicrobial, hypoglycemic and anti-tubercular activities.
An antitumor promoter from Moringa oleifera Lam.[Pubmed:10209341]
Mutat Res. 1999 Apr 6;440(2):181-8.
In the course of studies on the isolation of bioactive compounds from Philippine plants, the seeds of Moringa oleifera Lam. were examined and from the ethanol extract were isolated the new O-ethyl-4-(alpha-L-rhamnosyloxy)benzyl carbamate (1) together with seven known compounds, 4(alpha-L-rhamnosyloxy)-benzyl isothiocyanate (2), Niazimicin (3), niazirin (4), beta-sitosterol (5), glycerol-1-(9-octadecanoate) (6), 3-O-(6'-O-oleoyl-beta-D-glucopyranosyl)-beta-sitosterol (7), and beta-sitosterol-3-O-beta-D-glucopyranoside (8). Four of the isolates (2, 3, 7, and 8), which were obtained in relatively good yields, were tested for their potential antitumor promoting activity using an in vitro assay which tested their inhibitory effects on Epstein-Barr virus-early antigen (EBV-EA) activation in Raji cells induced by the tumor promoter, 12-O-tetradecanoyl-phorbol-13-acetate (TPA). All the tested compounds showed inhibitory activity against EBV-EA activation, with compounds 2, 3 and 8 having shown very significant activities. Based on the in vitro results, Niazimicin (3) was further subjected to in vivo test and found to have potent antitumor promoting activity in the two-stage carcinogenesis in mouse skin using 7,12-dimethylbenz(a)anthracene (DMBA) as initiator and TPA as tumor promoter. From these results, Niazimicin (3) is proposed to be a potent chemo-preventive agent in chemical carcinogenesis.


