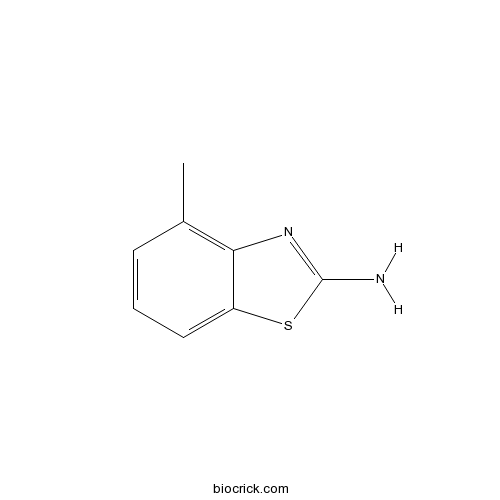
2-Amino-4-methylbenzothiazoleCAS# 1477-42-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1477-42-5 | SDF | Download SDF |
| PubChem ID | 15132 | Appearance | Powder |
| Formula | C8H8N2S | M.Wt | 164 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-methyl-1,3-benzothiazol-2-amine | ||
| SMILES | CC1=C2C(=CC=C1)SC(=N2)N | ||
| Standard InChIKey | GRIATXVEXOFBGO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H8N2S/c1-5-3-2-4-6-7(5)10-8(9)11-6/h2-4H,1H3,(H2,9,10) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2-Amino-4-methylbenzothiazole Dilution Calculator

2-Amino-4-methylbenzothiazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.0976 mL | 30.4878 mL | 60.9756 mL | 121.9512 mL | 152.439 mL |
| 5 mM | 1.2195 mL | 6.0976 mL | 12.1951 mL | 24.3902 mL | 30.4878 mL |
| 10 mM | 0.6098 mL | 3.0488 mL | 6.0976 mL | 12.1951 mL | 15.2439 mL |
| 50 mM | 0.122 mL | 0.6098 mL | 1.2195 mL | 2.439 mL | 3.0488 mL |
| 100 mM | 0.061 mL | 0.3049 mL | 0.6098 mL | 1.2195 mL | 1.5244 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- ZM 226600
Catalog No.:BCC6831
CAS No.:147695-92-9
- Magnolianin
Catalog No.:BCN3985
CAS No.:147663-91-0
- Calcipotriol monohydrate
Catalog No.:BCC1445
CAS No.:147657-22-5
- 5-Chloro-4-methoxy-2-oxo-1,2-dihydropyridine-3-carbonitrile
Catalog No.:BCC8744
CAS No.:147619-40-7
- Novobiocin Sodium
Catalog No.:BCC4812
CAS No.:1476-53-5
- ID-8
Catalog No.:BCC4787
CAS No.:147591-46-6
- DiMNF
Catalog No.:BCC3900
CAS No.:14756-24-2
- Racemodine
Catalog No.:BCN2023
CAS No.:147554-28-7
- trans-3-Hydroxycinnamic acid
Catalog No.:BCN5029
CAS No.:14755-02-3
- Bosentan
Catalog No.:BCC4640
CAS No.:147536-97-8
- Pitavastatin Calcium
Catalog No.:BCC3842
CAS No.:147526-32-7
- LY 288513
Catalog No.:BCC5772
CAS No.:147523-65-7
- WIN 18446
Catalog No.:BCC6273
CAS No.:1477-57-2
- 8-(3-Chlorostyryl)caffeine
Catalog No.:BCC7640
CAS No.:147700-11-6
- 6-O-(3'',4''-Dimethoxycinnamoyl)catalpol
Catalog No.:BCN1655
CAS No.:147714-71-4
- 3,4-Dimethoxybenzenepropanamine
Catalog No.:BCN1785
CAS No.:14773-42-3
- Fmoc-O-Phospho-Tyr-OH
Catalog No.:BCC3563
CAS No.:147762-53-6
- Repaglinide ethyl ester
Catalog No.:BCC9135
CAS No.:147770-06-7
- L-161,982
Catalog No.:BCC7393
CAS No.:147776-06-5
- DCG IV
Catalog No.:BCC5691
CAS No.:147782-19-2
- ω-Conotoxin MVIIC
Catalog No.:BCC5699
CAS No.:147794-23-8
- Santacruzamate A (CAY10683)
Catalog No.:BCC5488
CAS No.:1477949-42-0
- Cefcapene pivoxil hydrochloride
Catalog No.:BCC8906
CAS No.:147816-24-8
- Siramesine
Catalog No.:BCC4304
CAS No.:147817-50-3
Biotic and abiotic degradation of pesticide Dufulin in soils.[Pubmed:24323324]
Environ Sci Pollut Res Int. 2014 Mar;21(6):4331-42.
Dufulin is a newly developed antiviral agent (or pesticide) that activates systemic acquired resistance of plants. This pesticide is widely used in China to prevent abroad viral diseases in rice, tobacco and vegetables. In this study, the potential impacts such as soil type, moisture, temperature, and other factors on Dufulin degradation in soil were investigated. Degradation of Dufulin followed the first-order kinetics. The half-life values varied from 2.27 to 150.68 days. The dissipation of Dufulin was greatly affected by soil types, with DT50 (Degradation half time) varying between 17.59, 31.36, and 43.32 days for Eutric Gleysols, Cumulic Anthrosols, and Dystric Regosols, respectively. The elevated moisture accelerated the decay of Dufulin in soil. Degradation of Dufulin increased with temperature and its half-life values ranged from 16.66 to 42.79 days. Sterilization of soils and treatment with H2O2 resulted in a 6- and 8-fold decrease in degradation rates compared to the control, suggesting that Dufulin degradation was largely governed by microbial processes. Under different light spectra, the most effective degradation occurred with 100-W UV light (DT50=2.27 days), followed by 15-W UV light (DT50=8.32 days) and xenon light (DT50=14.26 days). Analysis by liquid chromatography-mass spectroscopy (LC-MS) revealed that 2-Amino-4-methylbenzothiazole was one of the major decayed products of Dufulin in soils, suggesting that elimination of diethyl phosphate and 2-fluorobenzaldehyde was most like the degradation pathway of Dufulin in Eutric Gleysols.
FTIR, FT-Raman, FT-NMR, UV-visible and quantum chemical investigations of 2-amino-4-methylbenzothiazole.[Pubmed:22226897]
Spectrochim Acta A Mol Biomol Spectrosc. 2012 Mar;88:220-31.
The FT-IR (4000-400 cm(-1)) and FT-Raman (4000-100 cm(-1)) spectral measurements and complete assignments of the observed spectra of 2-Amino-4-methylbenzothiazole (2A4MBT) have been proposed. Ab initio and DFT calculations have been performed and the structural parameters of the compound were determined from the optimised geometry with 6-31G(d,p), 6-311++G(d,p) and cc-pVDZ basis sets and giving energies, harmonic vibrational frequencies, depolarisation ratios, IR intensities and Raman activities. (1)H and (13)C NMR spectra were recorded and (1)H and (13)C nuclear magnetic resonance chemical shifts of the molecule were calculated using the gauge independent atomic orbital (GIAO) method. UV-visible spectrum of the compound was also recorded and the electronic properties, such as HOMO, LUMO and band gap energies were measured by time-dependent DFT (TD-DFT) approach. The geometric parameters, energies, harmonic vibrational frequencies, IR intensities, Raman activities chemical shifts and absorption wavelengths were compared with the available experimental data of the molecule. The influences of methyl and amino groups on the skeletal modes and on the proton chemical shifts have been investigated.
Experimental and theoretical surface enhanced Raman scattering study of 2-amino-4-methylbenzothiazole adsorbed on colloidal silver particles.[Pubmed:16853935]
J Phys Chem B. 2005 Dec 1;109(47):22536-44.
The adsorption of 2-Amino-4-methylbenzothiazole (2-AMBT) on colloidal silver particles has been investigated by a surface enhanced Raman scattering (SERS) study. The SERS spectra of the 2-AMBT molecule at varied adsorbate concentrations recorded in different time domains are compared with its Fourier transform infrared (FTIR) spectrum and normal Raman spectrum (NRS) in the bulk and in solution. The experimentally observed SERS spectra are compared with the theoretically modeled surface complexes using ab initio restricted Hatree-Fock (RHF) and density functional theory (DFT) calculations. The most favorable adsorptive sites of the 2-AMBT molecule have been estimated by natural population analysis (NPA) using the above-mentioned high level of theories. The enhancement of the in-plane modes together with the appearance of Ag-N stretching frequency at 215 cm(-1) indicates that the 2-AMBT molecule is adsorbed on the silver surface through the lone pair electrons of both nitrogen atoms with the molecular plane nearly vertical to the surface.


