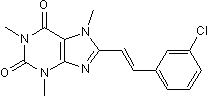
8-(3-Chlorostyryl)caffeineMAO-B inhibitor. Also A2A inhibitor CAS# 147700-11-6 |
- PI 828
Catalog No.:BCC7494
CAS No.:942289-87-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 147700-11-6 | SDF | Download SDF |
| PubChem ID | 147700-11-6 | Appearance | Powder |
| Formula | C16H15ClN4O2 | M.Wt | 330.77 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CSC | ||
| Solubility | Soluble to 25 mM in DMSO | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective adenosine A2A receptor antagonist and monoamine oxidase B (MAO-B) inhibitor (Ki values are 54 and 28200 nM at rat A2A and A1 receptors respectively and Ki ~ 100 nM at MAO-B). Potently protects against quinolinic acid-induced neuronal damage and is neuroprotective in the MPTP model of Parkinson's disease. |

8-(3-Chlorostyryl)caffeine Dilution Calculator

8-(3-Chlorostyryl)caffeine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0232 mL | 15.1162 mL | 30.2325 mL | 60.465 mL | 75.5812 mL |
| 5 mM | 0.6046 mL | 3.0232 mL | 6.0465 mL | 12.093 mL | 15.1162 mL |
| 10 mM | 0.3023 mL | 1.5116 mL | 3.0232 mL | 6.0465 mL | 7.5581 mL |
| 50 mM | 0.0605 mL | 0.3023 mL | 0.6046 mL | 1.2093 mL | 1.5116 mL |
| 100 mM | 0.0302 mL | 0.1512 mL | 0.3023 mL | 0.6046 mL | 0.7558 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- WIN 18446
Catalog No.:BCC6273
CAS No.:1477-57-2
- 2-Amino-4-methylbenzothiazole
Catalog No.:BCC8533
CAS No.:1477-42-5
- ZM 226600
Catalog No.:BCC6831
CAS No.:147695-92-9
- Magnolianin
Catalog No.:BCN3985
CAS No.:147663-91-0
- Calcipotriol monohydrate
Catalog No.:BCC1445
CAS No.:147657-22-5
- 5-Chloro-4-methoxy-2-oxo-1,2-dihydropyridine-3-carbonitrile
Catalog No.:BCC8744
CAS No.:147619-40-7
- Novobiocin Sodium
Catalog No.:BCC4812
CAS No.:1476-53-5
- ID-8
Catalog No.:BCC4787
CAS No.:147591-46-6
- DiMNF
Catalog No.:BCC3900
CAS No.:14756-24-2
- Racemodine
Catalog No.:BCN2023
CAS No.:147554-28-7
- trans-3-Hydroxycinnamic acid
Catalog No.:BCN5029
CAS No.:14755-02-3
- Bosentan
Catalog No.:BCC4640
CAS No.:147536-97-8
- 6-O-(3'',4''-Dimethoxycinnamoyl)catalpol
Catalog No.:BCN1655
CAS No.:147714-71-4
- 3,4-Dimethoxybenzenepropanamine
Catalog No.:BCN1785
CAS No.:14773-42-3
- Fmoc-O-Phospho-Tyr-OH
Catalog No.:BCC3563
CAS No.:147762-53-6
- Repaglinide ethyl ester
Catalog No.:BCC9135
CAS No.:147770-06-7
- L-161,982
Catalog No.:BCC7393
CAS No.:147776-06-5
- DCG IV
Catalog No.:BCC5691
CAS No.:147782-19-2
- ω-Conotoxin MVIIC
Catalog No.:BCC5699
CAS No.:147794-23-8
- Santacruzamate A (CAY10683)
Catalog No.:BCC5488
CAS No.:1477949-42-0
- Cefcapene pivoxil hydrochloride
Catalog No.:BCC8906
CAS No.:147816-24-8
- Siramesine
Catalog No.:BCC4304
CAS No.:147817-50-3
- Niazimicin
Catalog No.:BCN7641
CAS No.:147821-49-6
- Niazinin
Catalog No.:BCN7623
CAS No.:147821-57-6
Synthesis of (E)-8-(3-chlorostyryl)caffeine analogues leading to 9-deazaxanthine derivatives as dual A(2A) antagonists/MAO-B inhibitors.[Pubmed:23281824]
J Med Chem. 2013 Feb 14;56(3):1247-61.
A systematic modification of the caffeinyl core and substituents of the reference compound (E)-8-(3-Chlorostyryl)caffeine led to the 9-deazaxanthine derivative (E)-6-(4-chlorostyryl)-1,3,5,-trimethyl-1H-pyrrolo[3,2-d]pyrimidine-2,4-(3H,5H)-d ione (17f), which acts as a dual human A(2a) antagonist/MAO-B inhibitor (K(i)(A(2A)) = 260 nM; IC(50)(MAO-B) = 200 nM; IC(50)(MAO-A) = 10 muM) and dose dependently counteracts haloperidol-induced catalepsy in mice from 30 mg/kg by the oral route. The compound is the best balanced A(2A) antagonist/MAO-B inhibitor reported to date, and it could be considered as a new lead in the field of anti-Parkinson's agents. A number of analogues of 17f were synthesized and qualitative SARs are discussed. Two analogues of 17f, namely 18b and 19a, inhibit MAO-B with IC(50) of 68 and 48 nM, respectively, being 5-7-fold more potent than the prototypical MAO-B inhibitor deprenyl (IC(50) = 334 nM).
Inhibition of monoamine oxidase B by analogues of the adenosine A2A receptor antagonist (E)-8-(3-chlorostyryl)caffeine (CSC).[Pubmed:16442801]
Bioorg Med Chem. 2006 May 15;14(10):3512-21.
The adenosine A2A receptor has emerged as a possible target for the treatment of Parkinson's disease (PD). Evidence suggests that antagonism of the A2A receptor not only improves the symptoms of the disease but may also protect against the underlying degenerative processes. We have recently reported that several known adenosine A2A receptor antagonists (A2A antagonists) also are moderate to very potent inhibitors of monoamine oxidase B (MAO-B). The most potent among these was (E)-8-(3-Chlorostyryl)caffeine (CSC), a compound frequently used when examining the in vivo pharmacological effects of A2A antagonists. Since MAO-B inhibitors are also thought to possess antiparkinsonian properties, dual targeting drugs that block both MAO-B and A2A receptors may have enhanced therapeutic potential in the treatment of PD. In this study, we prepared selected analogues of CSC in an attempt to examine specific structural features that may be important for potent MAO-B inhibition. The results of a SAR study established that the potency of MAO-B inhibition by (E)-8-styrylcaffeinyl analogues depends upon the van der Waals volume (V(w)), lipophilicity (pi), and the Hammett constant (sigma(m)) of the substituents attached to C-3 of the phenyl ring of the styryl moiety. Potency also varies with substituents attached to C-4 with bulkiness (V(w)) and lipophilicity (pi) being the principal substituent descriptors.
(E)-8-(3-Chlorostyryl)-1,3,7-trimethylxanthine, a caffeine derivative acting both as antagonist of adenosine A2A receptors and as inhibitor of MAO-B.[Pubmed:16143772]
Acta Crystallogr C. 2005 Sep;61(Pt 9):o531-2.
In the crystal structure of (E)-8-(3-chlorostyryl)-1,3,7-trimethylxanthine (CSC) [systematic name: (E)-8-(3-chlorostyryl)-1,3,7-trimethyl-3,7-dihydro-1H-purine-2,6-dione], C16H15ClN4O2, the xanthine ring and the lateral styryl chain are coplanar. The crystal packing involves mainly parallel stacking of these planar molecules. The electrostatic potential calculated on the crystal structure conformation confirms the pharmacophore elements associated with MAO-B inhibition.
Effect of the adenosine A2A receptor antagonist 8-(3-chlorostyryl)caffeine on L-DOPA biotransformation in rat striatum.[Pubmed:14751592]
Brain Res. 2004 Feb 20;998(2):208-17.
In the present study, we investigated effects of the new selective adenosine A2A receptor antagonist 8-(3-Chlorostyryl)caffeine (CSC) on L-DOPA-induced dopamine (DA) release in the striatum of intact and reserpine-treated rats. CSC given in a pharmacologically effective dose of 5 mg/kg i.p. significantly increased striatal DA release after joint administration of L-DOPA (100 mg/kg, i.p.) and benserazide (50 mg/kg, i.p.) to intact and reserpine (2.5 mg/kg, s.c.)-injected rats. CSC did not change the elevated level of 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in intact rats, but raised it in DA-depleted animals. The availability of exogenous L-DOPA in the extracellular space was similar and equally increased by CSC in both intact and reserpinized rats. Our results suggest that the observed effects may be mediated by striatal adenosine A2A receptors, and are probably related to the CSC action on DA metabolism and the increased transport of exogenous L-DOPA into the brain. These observations might be of relevance, considering the use of selective A2A antagonists as potential supplements to L-DOPA therapy of Parkinson's disease.
8-(3-Chlorostyryl)caffeine may attenuate MPTP neurotoxicity through dual actions of monoamine oxidase inhibition and A2A receptor antagonism.[Pubmed:12130655]
J Biol Chem. 2002 Sep 27;277(39):36040-4.
Caffeine and more specific antagonists of the adenosine A(2A) receptor recently have been found to be neuroprotective in the MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) model of Parkinson's disease. Here we show that 8-(3-Chlorostyryl)caffeine (CSC), a specific A(2A) antagonist closely related to caffeine, also attenuates MPTP-induced neurotoxicity. Because the neurotoxicity of MPTP relies on its oxidative metabolism to the mitochondrial toxin MPP(+), we investigated the actions of CSC on striatal MPTP metabolism in vivo. CSC elevated striatal levels of MPTP but lowered levels of the oxidative intermediate MPDP(+) and of MPP(+), suggesting that CSC blocks the conversion of MPTP to MPDP(+) in vivo. In assessing the direct effects of CSC and A(2A) receptors on monoamine oxidase (MAO) activity, we found that CSC potently and specifically inhibited mouse brain mitochondrial MAO-B activity in vitro with a K(i) value of 100 nm, whereas caffeine and another relatively specific A(2A) antagonist produced little or no inhibition. The A(2A) receptor independence of MAO-B inhibition by CSC was further supported by the similarity of brain MAO activities derived from A(2A) receptor knockout and wild-type mice and was confirmed by demonstrating potent inhibition of A(2A) receptor knockout-derived MAO-B by CSC. Together, these data indicate that CSC possesses dual actions of MAO-B inhibition and A(2A) receptor antagonism, a unique combination suggesting a new class of compounds with the potential for enhanced neuroprotective properties.


