RutaecarpineCAS# 84-26-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 84-26-4 | SDF | Download SDF |
| PubChem ID | 65752 | Appearance | Yellow powder |
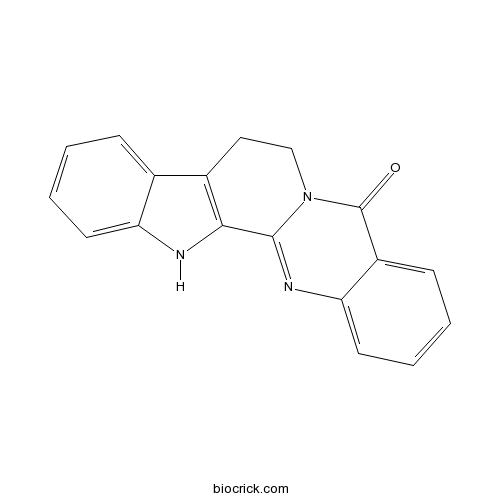
| Formula | C18H13N3O | M.Wt | 287.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Rutecarpine | ||
| Solubility | DMSO : 50 mg/mL (174.02 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| SMILES | C1CN2C(=NC3=CC=CC=C3C2=O)C4=C1C5=CC=CC=C5N4 | ||
| Standard InChIKey | ACVGWSKVRYFWRP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H13N3O/c22-18-13-6-2-4-8-15(13)20-17-16-12(9-10-21(17)18)11-5-1-3-7-14(11)19-16/h1-8,19H,9-10H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Rutaecarpine is an inhibitor of COX-2 with an IC50 value of 0.28 μM, and is also a potent inhibitor of CYP1A2. Rutaecarpine has anti-atherosclerosis, immunosuppressive, anti-inflammatory, gastroprotective, vasorelaxing, antihypertensive and anti-platelet effects. Rutaecarpine has positive inotropic and chronotropic effects on the guinea-pig isolated right atria, possible involvement of vanilloid receptors. Rutaecarpine may be useful in the prevention of ultraviolet A-induced photoaging, it inhibits ultraviolet A-induced reactive oxygen species generation, resulting in the enhanced expression of matrix metalloproteinase (MMP)-2 and MMP-9 in human skin cells. |
| Targets | NO | NOS | c-Myc | TRPV | LDL | P450 (e.g. CYP17) | IL Receptor | TNF-α | CGRP Receptor | Calcium Channel | MMP(e.g.TIMP) | gp120/CD4 | COX-2 | PGE | Phospholipase (e.g. PLA) |
| In vitro | Rutaecarpine inhibits angiotensin II-induced proliferation in rat vascular smooth muscle cells.[Pubmed: 23775171]Chin J Integr Med. 2014 Sep;20(9):682-7.OBJECTIVE: To evaluate the effects and possible mechanisms of Rutaecarpine on angiotensin II (Ang II)-induced proliferation in cultured rat vascular smooth muscle cells (VSMCs). METHODS: To examine the mechanisms involved in anti-proliferative effects of Rutaecarpine, nitric oxide (NO) levels and NO synthetase (NOS) activity were determined. Expressions of VSMC proliferation-related genes including endothelial nitric oxide synthase (eNOS), and c-myc hypertension related gene-1 (HRG-1) were determined by real-time reverse transcription-polymerase chain reaction (RT-PCR). RESULTS: Rutaecarpine (0.3-3.0 μmol/L) inhibited Ang II-induced VSMC proliferation and the best effects were achieved at 3.0 μmol/L. The Ang II-induced decreases in cellular NO contents and NOS activities were antagonized by Rutaecarpine (P <0.05). Ang II administration suppressed the expressions of eNOS and HRG-1, while increased c-myc expression (P <0.05). All these effects were attenuated by 3.0 μmol/L Rutaecarpine (P <0.05). CONCLUSION: Rutaecarpine is effective against Ang II-induced rat VSMC proliferation, and this effect is due, at least in part, to NO production and the modulation of VMSC proliferation-related gene expressions. The alkaloid rutaecarpine is a selective inhibitor of cytochrome P450 1A in mouse and human liver microsomes.[Pubmed: 11854157]Drug Metab Dispos. 2002 Mar;30(3):349-53.Rutaecarpine, evodiamine, and dehydroevodiamine are quinazolinocarboline alkaloids isolated from a traditional Chinese medicine, Evodia rutaecarpa. The in vitro effects of these alkaloids on cytochrome P450 (P450)-catalyzed oxidations were studied using mouse and human liver microsomes.
The vasorelaxing action of rutaecarpine: direct paradoxical effects on intracellular calcium concentration of vascular smooth muscle and endothelial cells.[Pubmed: 8786530]J Pharmacol Exp Ther. 1996 Mar;276(3):1016-21.We have examined both the hypotensive effect and the mechanism of intracellular Ca++ regulation, underlying Rutaecarpine (Rut)-induced vasodilatation. An i.v. bolus injection of Rut in anesthetized Sprague-Dawley rats produced a dose-dependent hypotensive effect.
|
| In vivo | Calcitonin gene-related peptide-mediated antihypertensive and anti-platelet effects by rutaecarpine in spontaneously hypertensive rats.[Pubmed: 18625276 ]Peptides. 2008 Oct;29(10):1781-8.We have previously reported that Chinese traditional medicine Rutaecarpine (Rut) produced a sustained hypotensive effect in phenol-induced and two-kidney, one-clip hypertensive rats. The aims of this study are to determine whether Rut could exert antihypertensive and anti-platelet effects in spontaneously hypertensive rats (SHR) and the underlying mechanisms.
Immunosuppressive effects of rutaecarpine in female BALB/c mice.[Pubmed: 16412592]Toxicol Lett. 2006 Jul 1;164(2):155-66.Rutaecarpine is a major quinazolinocarboline alkaloid isolated from Evodia rutaecarpa. It was reported to possess a wide spectrum of pharmacological activities, such as vasodilation, antithrombosis, and anti-inflammation.
A new class of COX-2 inhibitor, rutaecarpine from Evodia rutaecarpa.[Pubmed: 10669112 ]Inflamm Res. 1999 Dec;48(12):621-5.We investigated the effect of a new class of COX-2 inhibitor, Rutaecarpine, on the production of PGD2 in bone marrow derived mast cells (BMMC) and PGE2 in COX-2 transfected HEK293 cells. Inflammation was induced by lambda-carrageenan in male Splague-Dawley (SD) rats.
|
| Kinase Assay | Inhibition of UVA irradiation-modulated signaling pathways by rutaecarpine, a quinazolinocarboline alkaloid, in human keratinocytes.[Pubmed: 15363971 ]Rutaecarpine prevented dysfunction of endothelial gap junction induced by Ox-LDL via activation of TRPV1.[Pubmed: 25794845]Eur J Pharmacol. 2015 Jun 5;756:8-14.Gap junctions, which is formed by connexins, has been proved to play an important role in the atherogenesis development. Rutaecarpine was reported to inhibited monocyte migration, which indicates its potential for anti-atherosclerosis activity.
This study evaluated the effect of Rutaecarpine on endothelial dysfunction, and focused on the regulation of connexin expression in endothelial cells by Rutaecarpine.
Eur J Pharmacol. 2004 Sep 13;498(1-3):19-25.Matrix metalloproteinases (MMPs), a key component in photoaging of the skin due to exposure to ultraviolet A, appear to be increased by ultraviolet A irradiation-associated generation of reactive oxygen species.
|
| Cell Research | Effects of rutaecarpine on inflammatory cytokines in insulin resistant primary skeletal muscle cells.[Pubmed: 25423835]Zhongguo Zhong Yao Za Zhi. 2014 Aug;39(15):2930-5.
It is now well established that inflammation plays an important role in the development of numerous chronic metabolic diseases including insulin resistance (IR) and type 2 diabetes (T2DM). Skeletal muscle is responsible for 75% of total insulin-dependent glucose uptake; consequently, skeletal muscle IR is considered to be the primary defect of systemic IR development.
Our pre- vious study has shown that Rutaecarpine (Rut) can benefit blood lipid profile, mitigate inflammation, and improve kidney, liver, pan- creas pathology status of T2DM rats. However, the effects of Rut on inflammatory cytokines in the development of IR-skeletal muscle cells have not been studied. Thus, our objective was to investigate effects of Rut on inflammatory cytokines interleukiri (IL)-1, IL-6 and tumor necrosis factor (TNF)-α in insulin resistant primary skeletal muscle cells (IR-PSMC). |
| Animal Research | The protective effects of rutaecarpine on gastric mucosa injury in rats.[Pubmed: 15931578 ]Planta Med. 2005 May;71(5):416-9.Previous investigations have shown that calcitonin gene-related peptide (CGRP) protects gastric mucosa against injury induced by acetylsalicylic acid (ASA) and that Rutaecarpine activates vanilloid receptors to evoke CGRP release.
|

Rutaecarpine Dilution Calculator

Rutaecarpine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4807 mL | 17.4034 mL | 34.8068 mL | 69.6136 mL | 87.0171 mL |
| 5 mM | 0.6961 mL | 3.4807 mL | 6.9614 mL | 13.9227 mL | 17.4034 mL |
| 10 mM | 0.3481 mL | 1.7403 mL | 3.4807 mL | 6.9614 mL | 8.7017 mL |
| 50 mM | 0.0696 mL | 0.3481 mL | 0.6961 mL | 1.3923 mL | 1.7403 mL |
| 100 mM | 0.0348 mL | 0.174 mL | 0.3481 mL | 0.6961 mL | 0.8702 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Rutaecarpine, an alkaloid of Evodia rutaecarpa, is an inhibitor of COX-2 with an IC50 value of 0.28 μM.
In Vitro:Rutaecarpine has shown a variety of intriguing biological properties such as anti-thrombotic, anticancer, anti-inflammatory and analgesic, anti-obesity and thermoregulatory, vasorelaxing activity, as well as effects on the cardiovascular and endocrine systems[2]. Rutaecarpine inhibits COX-2 and COX-1 dependent phases of PGD2 generation in BMMC in a concentration-dependent manner with an IC50 of 0.28 μM and 8.7 μM, respectively. It inhibits COX-2-dependent conversion of exogenous arachidonic acid to PGE2 in a dose-dependent manner by the COX-2-transfected HEK293 cells[1].
In Vivo:Rutaecarpine showed in vivo anti-inflammatory activity on rat l-carrageenan induced paw edema by intraperitoneal administration[1]. Rutaecarpine significantly decreases the number of antibody-forming cells and causes weight decrease in spleen in a dose-dependent manner. In addition, rutaecarpine administered mice exhibit reduced splenic cellularity, decreased numbers of total T cells, CD4+ cells, CD8+ cells, and B cells in spleen. IL-2, interferon and IL-10 mRNA expressions are suppressed significantly by rutaecarpine treatment. The number of CD4+IL-2+ cells is reduced significantly following administration of mice with rutaecarpine[3].
References:
[1]. Moon TC, et al. A new class of COX-2 inhibitor, rutaecarpine from Evodia rutaecarpa. Inflamm Res. 1999 Dec;48(12):621-5.
[2]. Lee SH, et al. Progress in the studies on rutaecarpine. Molecules. 2008 Feb 6;13(2):272-300.
[3]. Jeon TW, et al. Immunosuppressive effects of rutaecarpine in female BALB/c mice.
Toxicol Lett. 2006 Jul 1;164(2):155-66.
- Hexestrol
Catalog No.:BCC4484
CAS No.:84-16-2
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
- 2,7-Dimethyl-1,4-dihydroxynaphthalene 1-O-glucoside
Catalog No.:BCN7611
CAS No.:839711-70-5
- Pluripotin
Catalog No.:BCC6178
CAS No.:839707-37-8
- GNF-7
Catalog No.:BCC6529
CAS No.:839706-07-9
- 4-Epicommunic acid
Catalog No.:BCN4382
CAS No.:83945-57-7
- Flavidinin
Catalog No.:BCN3599
CAS No.:83925-00-2
- Flavidin
Catalog No.:BCN6438
CAS No.:83924-98-5
- Mometasone furoate
Catalog No.:BCC4801
CAS No.:83919-23-7
- Isogomisin O
Catalog No.:BCN4381
CAS No.:83916-76-1
- 13-Hydroxylabda-8(17),14-dien-18-oic acid
Catalog No.:BCN1332
CAS No.:83915-59-7
- 12-Acetoxyabietic acid
Catalog No.:BCN4380
CAS No.:83905-81-1
- Ophiohayatone C
Catalog No.:BCN3608
CAS No.:84-33-3
- Syrosingopine
Catalog No.:BCN5365
CAS No.:84-36-6
- Stylopine
Catalog No.:BCN3715
CAS No.:84-39-9
- Tectoquinone
Catalog No.:BCN3481
CAS No.:84-54-8
- Anthraflavic acid
Catalog No.:BCC8831
CAS No.:84-60-6
- Anthraquinone
Catalog No.:BCC8832
CAS No.:84-65-1
- Diisobutyl phthalate
Catalog No.:BCN7148
CAS No.:84-69-5
- Dibutyl Phthalate
Catalog No.:BCC8411
CAS No.:84-74-2
- Lapachol
Catalog No.:BCN4391
CAS No.:84-79-7
- Vitamin K1
Catalog No.:BCN2209
CAS No.:84-80-0
- Xanthoxyletin
Catalog No.:BCN6579
CAS No.:84-99-1
- Fmoc-N-Me-Ala-OH
Catalog No.:BCC3210
CAS No.:84000-07-7
Antithrombotic effect of rutaecarpine, an alkaloid isolated from Evodia rutaecarpa, on platelet plug formation in in vivo experiments.[Pubmed:10930986]
Br J Haematol. 2000 Jul;110(1):110-5.
In this study, platelet thrombi formation was induced by irradiation of mesenteric venules with filtered light in mice pretreated intravenously with fluorescein sodium. Rutaecarpine (200 microg/g) significantly prolonged the latent period of inducing platelet plug formation in mesenteric venules when it was intravenously injected. Rutaecarpine (200 microg/g) prolonged occlusion time by approximately 1.5-fold (control 127 +/- 29 vs. taecarpine 188 +/- 23 s). Furthermore, aspirin (250 microg/g) also showed a similar prolongation of the occlusion time in this experiment. On a molar basis, Rutaecarpine was approximately twofold more potent than aspirin at prolonging the occlusion time. Furthermore, Rutaecarpine was also effective in reducing the mortality of ADP-induced acute pulmonary thromboembolism in mice when administered intravenously at doses of 25 and 50 microg/g. Intravenous injection of Rutaecarpine (50 microg/g) significantly prolonged the bleeding time by approximately 1.5-fold compared with normal saline in the severed mesenteric arteries of rats. Continuous infusion of Rutaecarpine (5 microg/g/min) also significantly increased the bleeding time 1. 5-fold, and the bleeding time returned to baseline within 60 min after cessation of Rutaecarpine infusion. These results suggest that Rutaecarpine has an effective anti-platelet effect in vivo and that it may be a potential therapeutic agent for arterial thrombosis, but it must be assessed further for toxicity.
The vasorelaxing action of rutaecarpine: direct paradoxical effects on intracellular calcium concentration of vascular smooth muscle and endothelial cells.[Pubmed:8786530]
J Pharmacol Exp Ther. 1996 Mar;276(3):1016-21.
We have examined both the hypotensive effect and the mechanism of intracellular Ca++ regulation, underlying Rutaecarpine (Rut)-induced vasodilatation. An i.v. bolus injection of Rut in anesthetized Sprague-Dawley rats produced a dose-dependent hypotensive effect. In isolated rat aorta rings, Rut (0.1-3 mu M) inhibited the phasic and tonic responses of norepinephrine- and phyenylephrine-induced contractions, respectively, mainly through an endothelium-dependent mechanism. However, the vasorelaxing effect of Rut (3 microM) persisted in denuded aorta, although to a much less extent than in intact tissue. As determined by the fura-2/AM (1-[2-(5-carboxyoxazol-2-yl)-6-aminobenzofuran-5-oxy]-2-(2'- amino-5'-methylphenoxy)-ethane-N,N,N,N-tetraacetic acid pentaacetoxymethyl ester) method, Rut (10 microM), in the presence of extracellular Ca++, suppressed the KCI-induced increment in the intracellular Ca++ concentration ([Ca++]i) of cultured vascular smooth muscle cells (VSMC). Rut (10 microM) also attenuated the norepinephrine-induced peak rise of [Ca++]i in VSMC placed in Ca++-free solution. On the other hand, Rut (1 and 10 microM) increased the level of [Ca++]i of cultured endothelial cells (EC) in the presence of extracellular Ca++. In conclusion, Rut acts on both VSMC and EC directly. In VSMC, it reduces [Ca++]i through the inhibition of Ca++ influx and Ca++ release from intracellular stores. In EC, Rut augments EC [Ca++]i by increasing Ca++ influx, possibly leading to nitric oxide release. The paradoxical regulation of Ca++ in both VSMC and EC acts simultaneously to cause vasorelaxation which could account, at least in part, for the hypotensive action. This is a most significant and a unique feature of this study.
Rutaecarpine prevented dysfunction of endothelial gap junction induced by Ox-LDL via activation of TRPV1.[Pubmed:25794845]
Eur J Pharmacol. 2015 Jun 5;756:8-14.
Gap junctions, which is formed by connexins, has been proved to play an important role in the atherogenesis development. Rutaecarpine was reported to inhibited monocyte migration, which indicates its potential for anti-atherosclerosis activity. This study evaluated the effect of Rutaecarpine on endothelial dysfunction, and focused on the regulation of connexin expression in endothelial cells by Rutaecarpine. Endothelia damage was induced by exposing HUVEC-12 to Ox-LDL (100mg/l) for 24h, which decreased the expression of protective proteins Cx37 and Cx40, but induced atherogenic Cx43 expression, in both mRNA and protein levels, concomitant with the impaired propidium iodide diffusion through the gap junctions. Pretreatment with Rutaecarpine effectively recovered the expression of Cx37 and Cx40, but inhibited Cx43 expression, thereby improving gap junction communication and significantly prevented the endothelial dysfunction. Consequently, the cell viability and nitric oxide production were increased, lactate dehydrogenase production was decreased and monocyte adhesion was inhibited. These protective effects of Rutaecarpine were remarkably attenuated by pretreatment with capsazepine, a competitive antagonist of transient receptor potential vanilloid subtype 1 (TRPV1). In summary, this study is the first to report that Rutaecarpine prevents endothelial injury and gap junction dysfunction induced by Ox-LDL in vitro, which is related to regulation of connexin expression patterns via TRPV1 activation. These results suggest that Rutaecarpine has the potential for use as an anti-atherosclerosis agent with a novel mechanism.
The alkaloid rutaecarpine is a selective inhibitor of cytochrome P450 1A in mouse and human liver microsomes.[Pubmed:11854157]
Drug Metab Dispos. 2002 Mar;30(3):349-53.
Rutaecarpine, evodiamine, and dehydroevodiamine are quinazolinocarboline alkaloids isolated from a traditional Chinese medicine, Evodia rutaecarpa. The in vitro effects of these alkaloids on cytochrome P450 (P450)-catalyzed oxidations were studied using mouse and human liver microsomes. Among these alkaloids, Rutaecarpine showed the most potent and selective inhibitory effect on CYP1A-catalyzed 7-methoxyresorufin O-demethylation (MROD) and 7-ethoxyresorufin O-deethylation (EROD) activities in untreated mouse liver microsomes. The IC(50) ratio of EROD to MROD was 6. For MROD activity, Rutaecarpine was a noncompetitive inhibitor with a K(i) value of 39 +/- 2 nM. In contrast, Rutaecarpine had no effects on benzo[a]pyrene hydroxylation (AHH), aniline hydroxylation, and nifedipine oxidation (NFO) activities. In human liver microsomes, 1 microM Rutaecarpine caused 98, 91, and 77% decreases of EROD, MROD, and phenacetin O-deethylation activities, respectively. In contrast, less than 15% inhibition of AHH, tolbutamide hydroxylation, chlorzoxazone hydroxylation, and NFO activities were observed in the presence of 1 microM Rutaecarpine. To understand the selectivity of inhibition of CYP1A1 and CYP1A2, inhibitory effects of Rutaecarpine were studied using liver microsomes of 3-methylcholanthrene (3-MC)-treated mice and Escherichia coli membrane expressing bicistronic human CYP1A1 and CYP1A2. Similar to the CYP1A2 inhibitor furafylline, Rutaecarpine preferentially inhibited MROD more than EROD and had no effect on AHH in 3-MC-treated mouse liver microsomes. For bicistronic human P450s, the IC(50) value of Rutaecarpine for EROD activity of CYP1A1 was 15 times higher than the value of CYP1A2. These results indicated that Rutaecarpine was a potent inhibitor of CYP1A2 in both mouse and human liver microsomes.
Calcitonin gene-related peptide-mediated antihypertensive and anti-platelet effects by rutaecarpine in spontaneously hypertensive rats.[Pubmed:18625276]
Peptides. 2008 Oct;29(10):1781-8.
We have previously reported that Chinese traditional medicine Rutaecarpine (Rut) produced a sustained hypotensive effect in phenol-induced and two-kidney, one-clip hypertensive rats. The aims of this study are to determine whether Rut could exert antihypertensive and anti-platelet effects in spontaneously hypertensive rats (SHR) and the underlying mechanisms. In vivo, SHR were given Rut and the blood pressure was monitored. Blood was collected for the measurements of calcitonin gene-related peptide (CGRP), tissue factor (TF) concentration and activity, and platelet aggregation, and the dorsal root ganglia were saved for examining CGRP expression. In vitro, the effects of Rut and CGRP on platelet aggregation were measured, and the effect of CGRP on platelet-derived TF release was also determined. Rut exerted a sustained hypotensive effect in SHR concomitantly with the increased synthesis and release of CGRP. The treatment of Rut also showed an inhibitory effect on platelet aggregation concomitantly with the decreased TF activity and TF antigen level in plasma. Study in vitro showed an inhibitory effect of Rut on platelet aggregation in the presence of thoracic aorta, which was abolished by capsazepine or CGRP(8-37), an antagonist of vanilloid receptor or CGRP receptor. Exogenous CGRP was able to inhibit both platelet aggregation and the release of platelet-derived TF, which were abolished by CGRP(8-37). The results suggest that Rut exerts both antihypertensive and anti-platelet effects through stimulating the synthesis and release of CGRP in SHR, and CGRP-mediated anti-platelet effect is related to inhibiting the release of platelet-derived TF.
A new class of COX-2 inhibitor, rutaecarpine from Evodia rutaecarpa.[Pubmed:10669112]
Inflamm Res. 1999 Dec;48(12):621-5.
OBJECTIVE AND DESIGN: We investigated the effect of a new class of COX-2 inhibitor, Rutaecarpine, on the production of PGD2 in bone marrow derived mast cells (BMMC) and PGE2 in COX-2 transfected HEK293 cells. Inflammation was induced by lambda-carrageenan in male Splague-Dawley (SD) rats. MATERIAL: Rutaecarpine (8,13-Dihydroindolo[2',3':3,4]pyridol[2,1-b]quinazolin -5(7H)-one) was isolated from the fruits of Evodia rutaecarpa. BMMC were cultured with WEHI-3 conditioned medium. c-Kit ligand and IL-10 were obtained by their expression in baculovirus. METHODS: The generation of PGD2 and PGE2 were determined by their assay kit. COX-1 and COX-2 protein and mRNA expression was determined by BMMC in the presence of KL, LPS and IL-10. TREATMENT: Rutaecarpine and indomethacin dissolved in 0.1% carboxymethyl cellulose was administered intraperitoneally and, 1 h later, lambda-carrageenan solution was injected to right hind paw of rats. Paw volumes were measured using plethysmometer 5 h after lambda-carrageenan injection. RESULTS: Rutaecarpine inhibited COX-2 and COX-1 dependent phases of PGD2 generation in BMMC in a concentration-dependent manner with an IC50 of 0.28 microM and 8.7 microM, respectively. It inhibited COX-2-dependent conversion of exogenous arachidonic acid to PGE2 in a dose-dependent manner by the COX-2-transfected HEK293 cells. However, Rutaecarpine inhibited neither PLA2 and COX-1 activity nor COX-2 protein and mRNA expression up to the concentration of 30 microM in BMMC, indicating that Rutaecarpine directly inhibited COX-2 activity. Furthermore, Rutaecarpine showed in vivo anti-inflammatory activity on rat lambda-carrageenan induced paw edema by intraperitoneal administration. CONCLUSION: Anti-inflammatory activity of Evodia rutaecarpa could be attributed at least in part by inhibition of COS-2.
Inhibition of UVA irradiation-modulated signaling pathways by rutaecarpine, a quinazolinocarboline alkaloid, in human keratinocytes.[Pubmed:15363971]
Eur J Pharmacol. 2004 Sep 13;498(1-3):19-25.
Matrix metalloproteinases (MMPs), a key component in photoaging of the skin due to exposure to ultraviolet A, appear to be increased by ultraviolet A irradiation-associated generation of reactive oxygen species. In this study, we investigated the effects of synthetic Rutaecarpine, which is also found in Evodia rutaecarpa, on the ultraviolet A-induced changes in the expression of gelatinases: matrix metalloproteinase (MMP)-2 and MMP-9 using HaCaT human keratinocytes as a model cellular system. Ultraviolet A irradiation of HaCaT cells increased the gelatinolytic activities of MMP-2 and MMP-9, which was significantly suppressed by the pretreatment with Rutaecarpine. In addition, Rutaecarpine significantly suppressed the ultraviolet A-induced enhanced expression of MMP-2 and MMP-9 proteins and mRNAs. Rutaecarpine also inhibited the H2O2-induced increase in the expression of MMP-2 and MMP-9. Furthermore, Rutaecarpine decreased the ultraviolet A-induced increased generation of reactive oxygen species. Taken together, these results suggest that Rutaecarpine inhibited ultraviolet A-induced reactive oxygen species generation, resulting in the enhanced expression of MMP-2 and MMP-9 in human skin cells. These results further suggest that ruetaecarpine may be useful in the prevention of ultraviolet A-induced photoaging.
Immunosuppressive effects of rutaecarpine in female BALB/c mice.[Pubmed:16412592]
Toxicol Lett. 2006 Jul 1;164(2):155-66.
Rutaecarpine is a major quinazolinocarboline alkaloid isolated from Evodia rutaecarpa. It was reported to possess a wide spectrum of pharmacological activities, such as vasodilation, antithrombosis, and anti-inflammation. In the present study, adverse effects of Rutaecarpine on immune functions were determined in female BALB/c mice. Rutaecarpine had no effects on hepatotoxicity parameters in mice, as measured by serum activities of aminotransferases. Meanwhile, Rutaecarpine significantly decreased the number of antibody-forming cells and caused weight decrease in spleen in a dose-dependent manner, when mice were administered with Rutaecarpine at 10mg/kg, 20mg/kg, 40 mg/kg or 80 cmg/kg once intravenously. In addition, Rutaecarpine administered mice exhibited reduced splenic cellularity, decreased numbers of total T cells, CD4(+) cells, CD8(+) cells, and B cells in spleen. IL-2, interferon-gamma and IL-10 mRNA expressions were suppressed significantly by Rutaecarpine treatment. The number of CD4(+)IL-2(+) cells was reduced significantly following administration of mice with Rutaecarpine. Furthermore, Rutaecarpine caused the cell cycle arrest in G(0)+G(1) phase in a dose-dependent manner. Rutaecarpine caused significant inductions of hepatic cytochrome P450 (CYP) 1A, 2B, and 2E1 activities dose-dependently. In the splenic lymphocyte proliferation assay, Rutaecarpine inhibited proliferation by LPS and Con A ex vivo, whereas it had no effects on in vitro proliferation. These results suggested that a single bolus intravenous injection of Rutaecarpine from 20mg/kg might cause immunosuppressive effects, and that Rutaecarpine-induced immunosuppression might be mediated, at least in part, through the inhibition of cytokine production and cell cycle arrest in G(0)+G(1) phase, and caused possibly by mechanisms associated with metabolic activation.
The protective effects of rutaecarpine on gastric mucosa injury in rats.[Pubmed:15931578]
Planta Med. 2005 May;71(5):416-9.
Previous investigations have shown that calcitonin gene-related peptide (CGRP) protects gastric mucosa against injury induced by acetylsalicylic acid (ASA) and that Rutaecarpine activates vanilloid receptors to evoke CGRP release. In the present study, we examined the protective effects of Rutaecarpine on gastric mucosa injury, and explored whether the protective effects of Rutaecarpine are related to stimulation of endogenous CGRP release via activating vanilloid receptors in rats. In an ASA-induced ulceration model, gastric mucosal ulcer index, pH value of gastric juice and plasma concentrations of CGRP were determined. ASA significantly increased the gastric mucosal ulcer index and the back-diffusion of H+ through the mucosa. Rutaecarpine at the doses of 100 or 300 microg/kg (i.v.), and 300 or 600 microg/kg (intragastric, i.g.) reduced the ulcer index and back-diffusion of H+, which was abolished by pretreatment with capsaicin (50 mg/kg, s.c.) or capsazepine (3 mg/kg, i.v.), a competitive vanilloid receptor antagonist. Rutaecarpine significantly increased the plasma concentration of CGRP, which was also abolished by capsazepine. In a stress-induced ulceration model, Rutaecarpine reduced gastric mucosal damages, which was abolished by capsazepine (5 mg/kg, i.p.). These results suggest that Rutaecarpine protects the gastric mucosa against injury induced by ASA and stress, and that the gastroprotective effect of Rutaecarpine is related to a stimulation of endogenous CGRP release via activation of the vanilloid receptor.
Rutaecarpine inhibits angiotensin II-induced proliferation in rat vascular smooth muscle cells.[Pubmed:23775171]
Chin J Integr Med. 2014 Sep;20(9):682-7.
OBJECTIVE: To evaluate the effects and possible mechanisms of Rutaecarpine on angiotensin II (Ang II)-induced proliferation in cultured rat vascular smooth muscle cells (VSMCs). METHODS: VSMCs were isolated from Male Sprague-Dawley rat aorta, and cultured by enzymic dispersion method. Experiments were performed with cells from passages 3-8. The cultured VSMCs were randomly divided into control, model (Ang II 0.1 mumol/L), and Rutaecarpine (0.3-3.0 mumol/L) groups. VMSC proliferation was induced by Ang II, and was evaluated by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay and cell counting. To examine the mechanisms involved in anti-proliferative effects of Rutaecarpine, nitric oxide (NO) levels and NO synthetase (NOS) activity were determined. Expressions of VSMC proliferation-related genes including endothelial nitric oxide synthase (eNOS), and c-myc hypertension related gene-1 (HRG-1) were determined by real-time reverse transcription-polymerase chain reaction (RT-PCR). RESULTS: Rutaecarpine (0.3-3.0 mumol/L) inhibited Ang II-induced VSMC proliferation and the best effects were achieved at 3.0 mumol/L. The Ang II-induced decreases in cellular NO contents and NOS activities were antagonized by Rutaecarpine (P <0.05). Ang II administration suppressed the expressions of eNOS and HRG-1, while increased c-myc expression (P <0.05). All these effects were attenuated by 3.0 mumol/L Rutaecarpine (P <0.05). CONCLUSION: Rutaecarpine is effective against Ang II-induced rat VSMC proliferation, and this effect is due, at least in part, to NO production and the modulation of VMSC proliferation-related gene expressions.
The positive inotropic and chronotropic effects of evodiamine and rutaecarpine, indoloquinazoline alkaloids isolated from the fruits of Evodia rutaecarpa, on the guinea-pig isolated right atria: possible involvement of vanilloid receptors.[Pubmed:11345696]
Planta Med. 2001 Apr;67(3):244-8.
Cardiotonic effects of evodiamine and Rutaecarpine, constituents of the fruits of Evodia rutaecarpa Bentham Rutaceae, were evaluated on guinea pig isolated atria. Comparison with capsaicin, a vanilloid receptor agonist, revealed similar positive inotropic and chronotropic activity, as judged from antagonistic effects of the competitive vanilloid receptor (capsaicin receptor) antagonist capsazepine, the non-competitive vanilloid receptor antagonist ruthenium red, the calcitonin gene related peptide antagonist CGRP(8-37), the P2X purinoceptor antagonist PPADS, and various desensitization studies. Evodiamine and Rutaecarpine produced transient positive inotropic and chronotropic effects on the guinea-pig isolated atria, followed by a desensitizing effect to additional administration. Dose-response relationships for evodiamine, Rutaecarpine and capsaicin were obtained. All the compounds evoked positive inotropic and chronotropic effects in a concentration-dependent manner. Maximal contractions for evodiamine, Rutaecarpine and capsaicin were observed at concentrations of 1 microM, 3 microM and 0.3 microM, respectively. The cardiotonic responses evoked by both evodiamine and Rutaecarpine were shifted to the right by capsazepine, an established antagonist of vanilloid receptor (capsaicin-receptor). The effects of both evodiamine (1 microM) and Rutaecarpine (3 microM) were abolished by pretreatment with a desensitizing dosage of capsaicin (1 microM), developing cross-tachyphylaxis between these compounds. The effects of evodiamine (1 microM), Rutaecarpine (3 microM) and capsaicin (0.3 microM) were also significantly reduced by pretreatment with ruthenium red (10 microM) and CGRP (8-37) (10 microM). The effects of evodiamine, Rutaecarpine and capsaicin were not affected by pretreatment with PPADS (100 microM), a highly selective P2X purinoceptor antagonist, and the possibility of the involvement of the P2X purinoceptor was excluded. These results suggest that the positive inotropic and chronotropic effects on the guinea-pig isolated right atria induced by both evodiamine and Rutaecarpine could be attributed to their interaction with vanilloid receptors and the resultant release of CGRP, a cardiotonic neurotransmitter, from capsaicin-sensitive nerves as with capsaicin.


