Dibutyl PhthalateCAS# 84-74-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 84-74-2 | SDF | Download SDF |
| PubChem ID | 3026 | Appearance | Powder |
| Formula | C16H22O4 | M.Wt | 278.3 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | Di-n-butyl phthalate | ||
| Solubility | Ethanol : ≥ 50 mg/mL (179.64 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | dibutyl benzene-1,2-dicarboxylate | ||
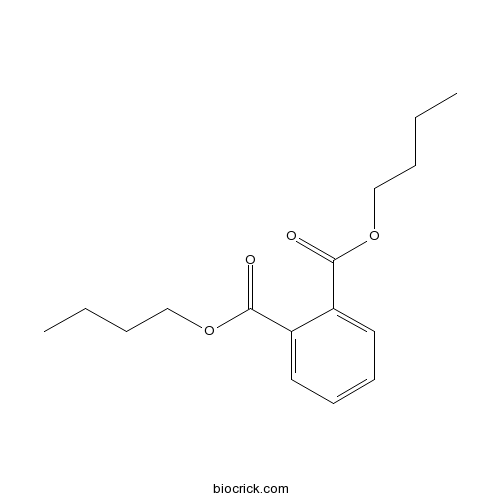
| SMILES | CCCCOC(=O)C1=CC=CC=C1C(=O)OCCCC | ||
| Standard InChIKey | DOIRQSBPFJWKBE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H22O4/c1-3-5-11-19-15(17)13-9-7-8-10-14(13)16(18)20-12-6-4-2/h7-10H,3-6,11-12H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1,2-Benzenedicarboxylic acid is one kind of allelochemical shows stronger allelopathic effect on itself than on wheat and pigeonpea. 1,2-Benzenedicarboxylic acid has antimicrobial activity, it shows anti-extended-spectrum beta-lactamases (ESBL) activity. |
| Targets | Antifection |
| In vitro | Antimicrobial activity of 1,2-benzenedicarboxylic acid, butyldecyl ester isolated from the seeds and pods of Acacia nilotica Linn[Reference: WebLink]Basic Research Journal of Microbiology, 2016 June, 3(2): 08-11.The antimicrobial activity of 1,2-Benzenedicarboxylic acid, butyldecyl ester isolated from Acacia nilotica was determined using standard methods. In-Silico Analysis of Streptomyces sp secondary metabolite 1,2-Benzenedicarboxylic acid, mono (2-ethylhexyl) Ester with ESBL proteins[Reference: WebLink]International Journal of Pharma & Bio Sciences, 2015 , 6 (1) :1190-1195.The anti-ESBL activity of 1,2-Benzenedicarboxylic acid, mono (2-ethylhexyl) ester (DMEHE) extracted from marine Streptomyces sp strain VITSJK8 was further confirmed by in silico analysis.
Identification of root exudation of Zea mays L. and allelopathy of 1,2-benzenedicarboxylic acid[Reference: WebLink]Journal of Gansu Agricultural University, 2007, 42(5):43-48.Root exudation is one of the most important resources of allelochemical.
|

Dibutyl Phthalate Dilution Calculator

Dibutyl Phthalate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5932 mL | 17.9662 mL | 35.9324 mL | 71.8649 mL | 89.8311 mL |
| 5 mM | 0.7186 mL | 3.5932 mL | 7.1865 mL | 14.373 mL | 17.9662 mL |
| 10 mM | 0.3593 mL | 1.7966 mL | 3.5932 mL | 7.1865 mL | 8.9831 mL |
| 50 mM | 0.0719 mL | 0.3593 mL | 0.7186 mL | 1.4373 mL | 1.7966 mL |
| 100 mM | 0.0359 mL | 0.1797 mL | 0.3593 mL | 0.7186 mL | 0.8983 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Diisobutyl phthalate
Catalog No.:BCN7148
CAS No.:84-69-5
- Anthraquinone
Catalog No.:BCC8832
CAS No.:84-65-1
- Anthraflavic acid
Catalog No.:BCC8831
CAS No.:84-60-6
- Tectoquinone
Catalog No.:BCN3481
CAS No.:84-54-8
- Stylopine
Catalog No.:BCN3715
CAS No.:84-39-9
- Syrosingopine
Catalog No.:BCN5365
CAS No.:84-36-6
- Ophiohayatone C
Catalog No.:BCN3608
CAS No.:84-33-3
- Rutaecarpine
Catalog No.:BCN4385
CAS No.:84-26-4
- Hexestrol
Catalog No.:BCC4484
CAS No.:84-16-2
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
- 2,7-Dimethyl-1,4-dihydroxynaphthalene 1-O-glucoside
Catalog No.:BCN7611
CAS No.:839711-70-5
- Pluripotin
Catalog No.:BCC6178
CAS No.:839707-37-8
- Lapachol
Catalog No.:BCN4391
CAS No.:84-79-7
- Vitamin K1
Catalog No.:BCN2209
CAS No.:84-80-0
- Xanthoxyletin
Catalog No.:BCN6579
CAS No.:84-99-1
- Fmoc-N-Me-Ala-OH
Catalog No.:BCC3210
CAS No.:84000-07-7
- Fmoc-N-Me-Val-OH
Catalog No.:BCC3358
CAS No.:84000-11-3
- Helioxanthin 8-1
Catalog No.:BCC5415
CAS No.:840529-13-7
- Lamotrigine
Catalog No.:BCC5051
CAS No.:84057-84-1
- Roquinimex
Catalog No.:BCC5355
CAS No.:84088-42-6
- 1-Benzhydrylpiperazine
Catalog No.:BCC8453
CAS No.:841-77-0
- Wilforlide A
Catalog No.:BCN4383
CAS No.:84104-71-2
- Wilforlide A acetate
Catalog No.:BCN4384
CAS No.:84104-80-3
- Triptotriterpenic acid A
Catalog No.:BCN6780
CAS No.:84108-17-8
The action mechanism and biocontrol potentiality of novel isolates of Saccharomyces cerevisiae against the aflatoxigenic Aspergillus flavus.[Pubmed:30554415]
Lett Appl Microbiol. 2019 Feb;68(2):104-111.
Inhibition of Aspergillus flavus growth and its aflatoxins production using the biocontrol agent Saccharomyces cerevisiae as well as to explore its mode of action was studied. Eight strains of S. cerevisiae strains were able to suppress the growth of A. flavus Z103. The maximum growth inhibition of A. flavus Z103 was obtained by living cells of S. cerevisiae EBF101 and S. cerevisiae 117 with 85 and 83%, respectively. The sporulation inhibition and hyphae deterioration of A. flavus Z103 by S. cerevisiae cells adhesion were observed under SEM; up to 99.8% inhibition of aflatoxins biosynthesis by A. flavus Z103 was resulted when the fungus was treated by autoclaved extracellular crude of S. cerevisiae. Also, the tested strains are potential to produce exo-chitinase which could be suggested as another mode of action for its antifungal activity. GC-MS analysis of S. cerevisiae 117 extracellular secondary metabolites revealed the existence of 4-Hydroxyphenethyl alcohol (46.32%), 4, 4-Dimethyloxazole (9.14%) and 1,2-Benzenedicarboxylic acid dioctyl ester (2.8%). Significance and Impact of the Study: The use of Saccharomyces cerevisiae instead of chemical preservatives in fermented food, animal and fish feed and storage cereal grains could encourage the food industry to produce organic food free of chemical additives. Overall, our data suggest the possibility of using S. cerevisiae as an alternative treatment in the food industries to control the dispersion and aflatoxins production by Aspergillus flavus during storage. This method could provide an additional probiotic effect in the digestive tract of consumers after ingestion of the treated food. So, our study clarifies the exact mechanisms responsible for the reduction of the aflatoxin contents by S. cerevisiae.


