Anthraflavic acidCAS# 84-60-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 84-60-6 | SDF | Download SDF |
| PubChem ID | 6776 | Appearance | Powder |
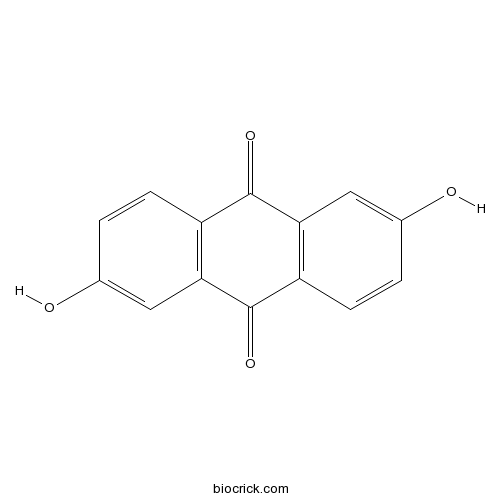
| Formula | C14H8O4 | M.Wt | 240 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2,6-dihydroxyanthracene-9,10-dione | ||
| SMILES | C1=CC2=C(C=C1O)C(=O)C3=C(C2=O)C=C(C=C3)O | ||
| Standard InChIKey | APAJFZPFBHMFQR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H8O4/c15-7-1-3-9-11(5-7)14(18)10-4-2-8(16)6-12(10)13(9)17/h1-6,15-16H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Anthraflavic acid Dilution Calculator

Anthraflavic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1667 mL | 20.8333 mL | 41.6667 mL | 83.3333 mL | 104.1667 mL |
| 5 mM | 0.8333 mL | 4.1667 mL | 8.3333 mL | 16.6667 mL | 20.8333 mL |
| 10 mM | 0.4167 mL | 2.0833 mL | 4.1667 mL | 8.3333 mL | 10.4167 mL |
| 50 mM | 0.0833 mL | 0.4167 mL | 0.8333 mL | 1.6667 mL | 2.0833 mL |
| 100 mM | 0.0417 mL | 0.2083 mL | 0.4167 mL | 0.8333 mL | 1.0417 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tectoquinone
Catalog No.:BCN3481
CAS No.:84-54-8
- Stylopine
Catalog No.:BCN3715
CAS No.:84-39-9
- Syrosingopine
Catalog No.:BCN5365
CAS No.:84-36-6
- Ophiohayatone C
Catalog No.:BCN3608
CAS No.:84-33-3
- Rutaecarpine
Catalog No.:BCN4385
CAS No.:84-26-4
- Hexestrol
Catalog No.:BCC4484
CAS No.:84-16-2
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
- 2,7-Dimethyl-1,4-dihydroxynaphthalene 1-O-glucoside
Catalog No.:BCN7611
CAS No.:839711-70-5
- Pluripotin
Catalog No.:BCC6178
CAS No.:839707-37-8
- GNF-7
Catalog No.:BCC6529
CAS No.:839706-07-9
- 4-Epicommunic acid
Catalog No.:BCN4382
CAS No.:83945-57-7
- Flavidinin
Catalog No.:BCN3599
CAS No.:83925-00-2
- Anthraquinone
Catalog No.:BCC8832
CAS No.:84-65-1
- Diisobutyl phthalate
Catalog No.:BCN7148
CAS No.:84-69-5
- Dibutyl Phthalate
Catalog No.:BCC8411
CAS No.:84-74-2
- Lapachol
Catalog No.:BCN4391
CAS No.:84-79-7
- Vitamin K1
Catalog No.:BCN2209
CAS No.:84-80-0
- Xanthoxyletin
Catalog No.:BCN6579
CAS No.:84-99-1
- Fmoc-N-Me-Ala-OH
Catalog No.:BCC3210
CAS No.:84000-07-7
- Fmoc-N-Me-Val-OH
Catalog No.:BCC3358
CAS No.:84000-11-3
- Helioxanthin 8-1
Catalog No.:BCC5415
CAS No.:840529-13-7
- Lamotrigine
Catalog No.:BCC5051
CAS No.:84057-84-1
- Roquinimex
Catalog No.:BCC5355
CAS No.:84088-42-6
- 1-Benzhydrylpiperazine
Catalog No.:BCC8453
CAS No.:841-77-0
Microbial Synthesis of Non-Natural Anthraquinone Glucosides Displaying Superior Antiproliferative Properties.[Pubmed:30154376]
Molecules. 2018 Aug 28;23(9). pii: molecules23092171.
Anthraquinones, naturally occurring bioactive compounds, have been reported to exhibit various biological activities, including anti-inflammatory, antiviral, antimicrobial, and anticancer effects. In this study, we biotransformed three selected anthraquinones into their novel O-glucoside derivatives, expressing a versatile glycosyltransferase (YjiC) from Bacillus licheniformis DSM 13 in Escherichia coli. Anthraflavic acid, alizarin, and 2-amino-3-hydroxyanthraquinone were exogenously fed to recombinant E. coli as substrate for biotransformation. The products Anthraflavic acid-O-glucoside, alizarin 2-O-beta-d-glucoside, and 2-amino-3-O-glucosyl anthraquinone produced in the culture broths were characterized by various chromatographic and spectroscopic analyses. The comparative anti-proliferative assay against various cancer cells (gastric cancer-AGS, uterine cervical cancer-HeLa, and liver cancer-HepG2) were remarkable, since the synthesized glucoside compounds showed more than 60% of cell growth inhibition at concentrations ranging from ~50 muM to 100 muM. Importantly, one of the synthesized glucoside derivatives, alizarin 2-O-glucoside inhibited more than 90% of cell growth in all the cancer cell lines tested.
Inhibition and active sites of UDP-glucuronosyltransferases 2B7 and 1A1.[Pubmed:12433804]
Drug Metab Dispos. 2002 Dec;30(12):1364-7.
Two human UDP-glucuronosyltransferases (UGTs), UGT2B7 and UGT1A1, catalyze the glucuronidation of many endo- and xenobiotics. Although UGT1A1 uniquely catalyzes the glucuronidation of the endobiotic, bilirubin, and UGT2B7 uniquely catalyzes the glucuronidation of morphine to both the 3-0 glucuronide and the 6-0 glucuronide, both catalyze the glucuronidation of the mixed opioid agonist/antagonist buprenorphine with high efficiency. Etonitazenyl, a mu opioid receptor antagonist, was found to inhibit competitively opioid, steroid, and other substrate glucuronidation reactions catalyzed by UGT2B7. Data showing several benzodiazepines and alternative substrates interacting competitively support previous work, which indicates a single binding domain within UGT2B7. Etonitazenyl also competitively inhibited the glucuronidation of buprenorphine catalyzed by UGT1A1. However, neither etonitazenyl nor buprenorphine inhibited bilirubin glucuronidation except at very high concentrations. Therefore, it is unlikely that buprenorphine therapy for opioid or other drug addiction would influence bilirubin glucuronidation and lead to hyperbilirubenmia. Anthraflavic acid and catechol estrogen glucuronidation, catalyzed by UGT1A1, was also not inhibited by etonitazenyl or buprenorphine. Reactions catalyzed by UGT1A6 were not affected by etonitazenyl. These studies indicate that UGT2B7 has one binding site and that UGT1A1 has two or more binding sites for xenobiotics and endobiotics.
Differential modulation of UDP-glucuronosyltransferase 1A1 (UGT1A1)-catalyzed estradiol-3-glucuronidation by the addition of UGT1A1 substrates and other compounds to human liver microsomes.[Pubmed:12386134]
Drug Metab Dispos. 2002 Nov;30(11):1266-73.
Previous results demonstrating homotropic activation of human UDP-glucuronosyltransferase (UGT) 1A1-catalyzed estradiol-3-glucuronidation led us to investigate the effects of 16 compounds on estradiol glucuronidation by human liver microsomes (HLM). In confirmation of previous work using alamethicin-treated HLM pooled from four livers, UGT1A1-catalyzed estradiol-3-glucuronidation demonstrated homotropic activation kinetics (S(50) = 22 microM, Hill coefficient, n = 1.9) whereas estradiol-17-glucuronidation (catalyzed by other UGT enzymes) followed Michaelis-Menten kinetics (K(m) = 7 microM). Modulatory effects of the following compounds were investigated: bilirubin, eight flavonoids, 17alpha-ethynylestradiol (17alpha-EE), estriol, 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine (PhIP), Anthraflavic acid, retinoic acid, morphine, and ibuprofen. Although the classic UGT1A1 substrate bilirubin was a weak competitive inhibitor of estradiol-3-glucuronidation, the estrogens and Anthraflavic acid activated or inhibited estradiol-3-glucuronidation dependent on substrate and effector concentrations. For example, at substrate concentrations of 5 and 10 microM, estradiol-3-glucuronidation activity was stimulated by as much as 80% by low concentrations of 17alpha-EE but was unaltered by flavanone. However, at higher substrate concentrations (25-100 microM) estradiol-3-glucuronidation was inhibited by about 55% by both compounds. Anthraflavic acid and PhIP were also stimulators of estradiol 3-glucuronidation at low substrate concentrations. The most potent inhibitor of estradiol 3-glucuronidation was the flavonoid tangeretin. The UGT2B7 substrates morphine and ibuprofen had no effect on estradiol 3-glucuronidation, whereas retinoic acid was slightly inhibitory. Estradiol-17-glucuronidation was inhibited by 17alpha-EE, estriol, and naringenin but was not activated by any compound. This study demonstrates that the interactions of substrates and inhibitors at the active site of UGT1A1 are complex, yielding both activation and competitive inhibition kinetics.


