Ro 61-8048Potent kynurenine 3-monooxygenase (KMO) inhibitor CAS# 199666-03-0 |
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- WHI-P180 hydrochloride
Catalog No.:BCC4243
CAS No.:153437-55-9
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- RGB-286638
Catalog No.:BCC5519
CAS No.:784210-87-3
- AT7519 Hydrochloride
Catalog No.:BCC1376
CAS No.:902135-91-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 199666-03-0 | SDF | Download SDF |
| PubChem ID | 5282337 | Appearance | Powder |
| Formula | C17H15N3O6S2 | M.Wt | 421.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 59 mg/mL (139.99 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
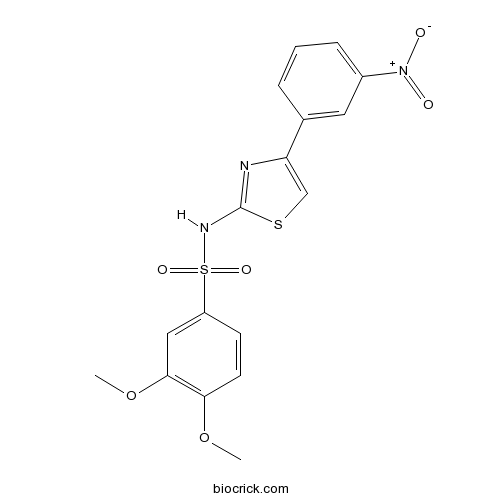
| Chemical Name | 3,4-dimethoxy-N-[4-(3-nitrophenyl)-1,3-thiazol-2-yl]benzenesulfonamide | ||
| SMILES | COC1=C(C=C(C=C1)S(=O)(=O)NC2=NC(=CS2)C3=CC(=CC=C3)[N+](=O)[O-])OC | ||
| Standard InChIKey | NDPBMCKQJOZAQX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H15N3O6S2/c1-25-15-7-6-13(9-16(15)26-2)28(23,24)19-17-18-14(10-27-17)11-4-3-5-12(8-11)20(21)22/h3-10H,1-2H3,(H,18,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and competitive kynurenine 3-monooxygenase (kynurenine 3-hydroxylase; KMO) inhibitor (Ki = 4.8 nM, IC50 = 37 nM). Increases kynurenic acid levels to concentrations that antagonize the glycine site of NMDA receptors. Brain penetrant and exhibits antidystonic, anticonvulsant and neuroprotective activities. |

Ro 61-8048 Dilution Calculator

Ro 61-8048 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3728 mL | 11.8638 mL | 23.7276 mL | 47.4552 mL | 59.319 mL |
| 5 mM | 0.4746 mL | 2.3728 mL | 4.7455 mL | 9.491 mL | 11.8638 mL |
| 10 mM | 0.2373 mL | 1.1864 mL | 2.3728 mL | 4.7455 mL | 5.9319 mL |
| 50 mM | 0.0475 mL | 0.2373 mL | 0.4746 mL | 0.9491 mL | 1.1864 mL |
| 100 mM | 0.0237 mL | 0.1186 mL | 0.2373 mL | 0.4746 mL | 0.5932 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ro 61-8048 is a potent and selective inhibitor of kynurenine hydroxylase with IC50 of 37 nM. IC50 value: 37 nM [1] Target: kynurenine hydroxylase inhibitor in vitro: in vivo: Ro 61-8048 blocked rat and gerbil kynurenine 3-hydroxylase after oral administration, with ED50's in the 3-5 mumol/kg range in gerbil brain. In a microdialysis experiment in rats, 16 dose dependently increased kynurenic acid concentration in the extracellular hippocampal fluid. A dose of 100 mumol/kg po led to a 7.5-fold increase in kynurenic acid outflow [1]. A significant reduction in infarct volumes also was found when the kynurenine hydroxylase inhibitors were given to rats after permanent middle cerebral artery occlusion (from 207+/-111 mm3 in vehicle-treated rats to 82+/-18 and to 62+/-57 mm3 in rats treated with mNBA, 400 mg/kg intraperitoneally, or with Ro 61-8048, 40 mg/kg intraperitoneally, respectively) [2]. intrastriatal injections of Ro 61-8048 (60-80 microg/hemisphere) significantly reduced the severity of dystonia in dt(sz) hamsters [3].
References:
[1]. Rover S, et al. Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl)benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. J Med Chem. 1997 Dec 19;40(26):4378-85.
[2]. Cozzi A, et al. Kynurenine hydroxylase inhibitors reduce ischemic brain damage: studies with (m-nitrobenzoyl)-alanine (mNBA) and 3,4-dimethoxy-[-N-4-(nitrophenyl)thiazol-2yl]-benzenesulfonamide (Ro 61-8048) in models of focal or global brain ischemia. J C
[3]. Hamann M, et al. Effects of the kynurenine 3-hydroxylase inhibitor Ro 61-8048 after intrastriatal injections on the severity of dystonia in the dt sz mutant. Eur J Pharmacol. 2008 May 31;586(1-3):156-9.
- CP 465022 hydrochloride
Catalog No.:BCC7520
CAS No.:199655-36-2
- JIB-04
Catalog No.:BCC4548
CAS No.:199596-05-9
- Methyllucidone
Catalog No.:BCN4877
CAS No.:19956-54-8
- Lucidone
Catalog No.:BCN4876
CAS No.:19956-53-7
- NNC 26-9100
Catalog No.:BCC7361
CAS No.:199522-35-5
- 2-Amino-4-chlorobenzothiazole
Catalog No.:BCC8529
CAS No.:19952-47-7
- Veraguensin
Catalog No.:BCN2163
CAS No.:19950-55-1
- Y-33075
Catalog No.:BCC2064
CAS No.:199433-58-4
- 3-Epicabraleadiol
Catalog No.:BCN4875
CAS No.:19942-04-2
- Gymnestrogenin
Catalog No.:BCN7846
CAS No.:19942-02-0
- Kolavenol
Catalog No.:BCN4680
CAS No.:19941-83-4
- Crocatone
Catalog No.:BCN3532
CAS No.:19937-86-1
- LY 334370 hydrochloride
Catalog No.:BCC7559
CAS No.:199673-74-0
- Liguiritigenin-7-O-D-apiosyl-4'-O-D-glucoside
Catalog No.:BCN2840
CAS No.:199796-12-8
- 1-Actamido-3,5-dimethyladmantane
Catalog No.:BCC8449
CAS No.:19982-07-1
- Stattic
Catalog No.:BCC1176
CAS No.:19983-44-9
- PD 166793
Catalog No.:BCC2376
CAS No.:199850-67-4
- Isocudraniaxanthone B
Catalog No.:BCN6887
CAS No.:199851-52-0
- RS 127445
Catalog No.:BCC1909
CAS No.:199864-87-4
- DL-Alanyl-DL-Methionine
Catalog No.:BCC8950
CAS No.:1999-43-5
- Chrysoeriol-7-O-glucoside
Catalog No.:BCN3796
CAS No.:19993-32-9
- UCL 1684
Catalog No.:BCC7016
CAS No.:199934-16-2
- CVT-313
Catalog No.:BCC1503
CAS No.:199986-75-9
- ALX 5407 hydrochloride
Catalog No.:BCC7168
CAS No.:200006-08-2
Effects of the kynurenine 3-hydroxylase inhibitor Ro 61-8048 after intrastriatal injections on the severity of dystonia in the dt sz mutant.[Pubmed:18353306]
Eur J Pharmacol. 2008 May 31;586(1-3):156-9.
Striatal dysfunctions seem to play a key role in the pathophysiology of dystonia in the dt(sz) mutant hamster, a model of paroxysmal non-kinesigenic dyskinesia, in which stress precipitates dystonic episodes. Previous examinations have shown changes in kynurenic acid levels and antidystonic effects of the kynurenine 3-hydroxylase inhibitor 3,4-dimethoxy-N-[4-(3-nitrophenyl)thiazol-2-yl]benzenesulfon-amide (Ro 61-8048) after systemic treatment in dt(sz) hamsters. In the present study, intrastriatal injections of Ro 61-8048 (60-80 microg/hemisphere) significantly reduced the severity of dystonia in dt(sz) hamsters, suggesting that kynurenine 3-hydroxylase inhibitors may be interesting candidates for managing dyskinesias which are related to striatal dysfunction.
Metabolism and pharmacokinetics of JM6 in mice: JM6 is not a prodrug for Ro-61-8048.[Pubmed:22942319]
Drug Metab Dispos. 2012 Dec;40(12):2297-306.
Understanding whether regulation of tryptophan metabolites can ameliorate neurodegeneration is of high interest to investigators. A recent publication describes 3,4-dimethoxy-N-(4-(3-nitrophenyl)-5-(piperidin-1-ylmethyl)thiazol-2-yl)benzenesu lfonamide (JM6) as a novel prodrug for the kynurenine 3-monooxygenase (KMO) inhibitor 3,4-dimethoxy-N-(4-(3-nitrophenyl)thiazol-2-yl)benzenesulfonamide (Ro-61-8048) that elicits therapeutic effects in mouse models of Huntington's and Alzheimer's diseases (Cell 145:863-874, 2011). Our evaluation of the metabolism and pharmacokinetics of JM6 and Ro-61-8048 indicate instead that Ro-61-8048 concentrations in mouse plasma after JM6 administration originate from a Ro-61-8048 impurity (<0.1%) in JM6. After a 0.05 mg/kg Ro-61-8048 oral dose alone or coadministered with 10 mg/kg JM6 to mice, the Ro-61-8048 areas under the concentration-time curves (AUCs) from 0 to infinity were similar (4300 and 4900 nM x h, respectively), indicating no detectable contributions of JM6 metabolism to the Ro-61-8048 AUCs. JM6 was stable in incubations under acidic conditions and Ro-61-8048 was not a product of JM6 metabolism in vitro (plasma, blood, or hepatic models). Species differences in the quantitative rate of oxidative metabolism indicate that major circulating JM6 metabolite(s) in mice are unlikely to be major in humans: JM6 is rapidly metabolized via the piperidyl moiety in mouse (forming an iminium ion reactive intermediate) but is slowly metabolized in human (in vitro), primarily via O-dealkylation at the phenyl ring. Our data indicate that JM6 is not a prodrug for Ro-61-8048 and is not a potent KMO inhibitor.
Implication of NMDA receptors in the antidyskinetic activity of cabergoline, CI-1041, and Ro 61-8048 in MPTP monkeys with levodopa-induced dyskinesias.[Pubmed:18704766]
J Mol Neurosci. 2009 Jun;38(2):128-42.
This study assessed striatal N-methyl-D-aspartate (NMDA) glutamate receptors of 1-methyl 4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) monkeys with levodopa (L-DOPA)-induced dyskinesias (LID). In a first experiment, four MPTP monkeys receiving L-DOPA/Benserazide alone developed dyskinesias. Four MPTP monkeys received L-DOPA/Benserazide plus CI-1041 an NMDA antagonist selective for NR1/NR2B and four were treated with L-DOPA/Benserazide plus a small dose of cabergoline; one monkey of each group developed mild dyskinesias at the end of treatment. In a second experiment, a kynurenine 3-hydroxylase inhibitor Ro 61-8048, combined with L-DOPA/Benserazide, reduced dyskinesias in MPTP monkeys. Drug-treated MPTP monkeys were compared to intact monkeys and saline-treated MPTP monkeys. Glutamate receptors were investigated by autoradiography using [(3)H]CGP-39653 (NR1/NR2A antagonist) and [(3)H]Ro25-6981 (NR1/NR2B antagonist). In general, striatal [(3)H]CGP-39653 specific binding was unaltered in all experimental groups. MPTP lesion decreased striatal [(3)H]Ro25-6981 specific binding; these levels were enhanced in the L-DOPA-alone-treated MPTP monkeys and decreased in antidyskinetic drugs treated monkeys. Maximal dyskinesias scores of the MPTP monkeys correlated significantly with [(3)H]Ro25-6981 specific binding in the rostral and caudal striatum. Hence, MPTP lesion, L-DOPA treatment and prevention of LID with CI-1041 and cabergoline, or reduction with Ro 61-8048 were associated with modulation of NR2B/NMDA glutamate receptors.
Reducing cannabinoid abuse and preventing relapse by enhancing endogenous brain levels of kynurenic acid.[Pubmed:24121737]
Nat Neurosci. 2013 Nov;16(11):1652-61.
In the reward circuitry of the brain, alpha-7-nicotinic acetylcholine receptors (alpha7nAChRs) modulate effects of Delta(9)-tetrahydrocannabinol (THC), marijuana's main psychoactive ingredient. Kynurenic acid (KYNA) is an endogenous negative allosteric modulator of alpha7nAChRs. Here we report that the kynurenine 3-monooxygenase (KMO) inhibitor Ro 61-8048 increases brain KYNA levels and attenuates cannabinoid-induced increases in extracellular dopamine in reward-related brain areas. In the self-administration model of drug abuse, Ro 61-8048 reduced the rewarding effects of THC and the synthetic cannabinoid WIN 55,212-2 in squirrel monkeys and rats, respectively, and it also prevented relapse to drug-seeking induced by reexposure to cannabinoids or cannabinoid-associated cues. The effects of enhancing endogenous KYNA levels with Ro 61-8048 were prevented by positive allosteric modulators of alpha7nAChRs. Despite a clear need, there are no medications approved for treatment of marijuana dependence. Modulation of KYNA offers a pharmacological strategy for achieving abstinence from marijuana and preventing relapse.
Kynurenine 3-mono-oxygenase inhibitors attenuate post-ischemic neuronal death in organotypic hippocampal slice cultures.[Pubmed:12354294]
J Neurochem. 2002 Sep;82(6):1465-71.
Kynurenine 3-mono-oxygenase (KMO) inhibitors reduce 3-hydroxykynurenine (3-HK) and quinolinic acid (QUIN) neosynthesis and facilitate kynurenine metabolism towards kynurenic acid (KYNA) formation. They also reduce tissue damage in models of focal or transient global cerebral ischemia in vivo. We used organotypic hippocampal slice cultures exposed to oxygen and glucose deprivation (OGD) to investigate KMO mechanism(s) of neuroprotective activity. Exposure of the slices to 30 min of OGD caused CA1 pyramidal cell death and significantly decreased the amount of KYNA released in the incubation medium. The KMO inhibitors (m-nitrobenzoyl)-alanine (30-100 micro m) or 3,4-dimethoxy-[-N-4-(nitrophenyl)thiazol-2yl]-benzenesulfonamide (1-10 micro m) reduced post-ischemic neuronal death and increased KYNA concentrations in slice incubation media. The maximal concentration of KYNA detected in the incubation media of slices treated with KMO inhibitors was approximately 50 nm and was too low to efficiently interact with alpha7 nicotinic acetylcholine receptors or with the glycineb site of N-methyl-d-aspartate (NMDA) receptors. On the other hand, the addition of either 3-HK or QUIN (1-10 micro m) to OGD-exposed hippocampal slices prevented the neuroprotective activity of KMO inhibitors. Our results suggest that KMO inhibitors reduce the neuronal death found in the CA1 region of organotypic hippocampal slices exposed to 30 min of OGD by decreasing the local synthesis of 3-HK and QUIN.
Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl)benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase.[Pubmed:9435907]
J Med Chem. 1997 Dec 19;40(26):4378-85.
In this paper we describe the synthesis, structure-activity relationship (SAR), and biochemical characterization of N-(4-phenylthiazol-2-yl)benzenesulfonamides as inhibitors of kynurenine 3-hydroxylase. The compounds 3,4-dimethoxy-N-[4-(3-nitrophenyl)thiazol-2-yl]benzenesulfonamide 16 (IC50 = 37 nM, Ro-61-8048) and 4-amino-N-[4-[2-fluoro-5-(trifluoromethyl)phenyl]-thiazol-2-yl] benzenesulfonamide 20 (IC50 = 19 nM) were found to be high-affinity inhibitors of this enzyme in vitro. In addition, both compounds blocked rat and gerbil kynurenine 3-hydroxylase after oral administration, with ED50's in the 3-5 mumol/kg range in gerbil brain. In a microdialysis experiment in rats, 16 dose dependently increased kynurenic acid concentration in the extracellular hippocampal fluid. A dose of 100 mumol/kg po led to a 7.5-fold increase in kynurenic acid outflow. These new compounds should allow detailed investigation of the pathophysiological role of the kynurenine pathway after neuronal injury.


