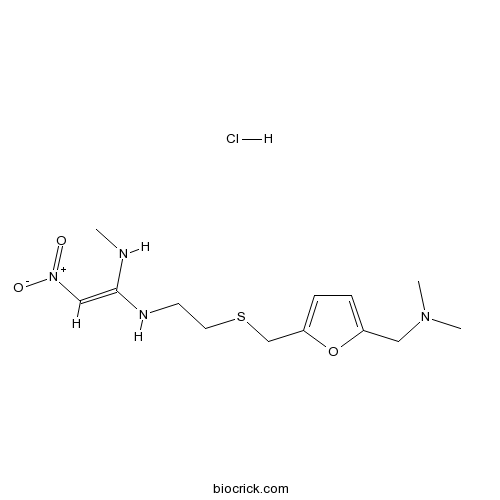
Ranitidine HydrochlorideHistamine H2-receptor antagonist CAS# 66357-59-3 |
- Tolbutamide
Catalog No.:BCC5001
CAS No.:64-77-7
- Melatonin
Catalog No.:BCN2196
CAS No.:73-31-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 66357-59-3 | SDF | Download SDF |
| PubChem ID | 3033332 | Appearance | Powder |
| Formula | C13H23ClN4O3S | M.Wt | 350.86 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 50 mg/mL (142.51 mM) DMSO : 50 mg/mL (142.51 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | N-[2-[[[5-[(Dimethylamino)methyl]-2 | ||
| SMILES | [H+].[Cl-].CNC(/NCCSCc1oc(CN(C)C)cc1)=C[N+]([O-])=O | ||
| Standard InChIKey | GGWBHVILAJZWKJ-KJEVSKRMSA-N | ||
| Standard InChI | InChI=1S/C13H22N4O3S.ClH/c1-14-13(9-17(18)19)15-6-7-21-10-12-5-4-11(20-12)8-16(2)3;/h4-5,9,14-15H,6-8,10H2,1-3H3;1H/b13-9+; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, selective and competitive histamine H2 receptor antagonist (pA2 = 6.95 - 7.2). Inhibits gastric acid secretion induced by histamine, pentagastrin, bethanecol and food in vivo. Also inhibits aspirin-induced gastric lesions. |

Ranitidine Hydrochloride Dilution Calculator

Ranitidine Hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8501 mL | 14.2507 mL | 28.5014 mL | 57.0028 mL | 71.2535 mL |
| 5 mM | 0.57 mL | 2.8501 mL | 5.7003 mL | 11.4006 mL | 14.2507 mL |
| 10 mM | 0.285 mL | 1.4251 mL | 2.8501 mL | 5.7003 mL | 7.1253 mL |
| 50 mM | 0.057 mL | 0.285 mL | 0.57 mL | 1.1401 mL | 1.4251 mL |
| 100 mM | 0.0285 mL | 0.1425 mL | 0.285 mL | 0.57 mL | 0.7125 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ranitidine is a histamine H2-receptor antagonist that inhibits stomach acid production.Ranitidine (Zantac) is a histamine H2-receptor antagonist with IC50 of 3.3 ± 1.4 uM. It inhibits stomach acid production. It is also used alongside fexofenadine and oth
- Ranitidine
Catalog No.:BCC9134
CAS No.:66357-35-5
- 5'-Deoxy-5-fluorocytidine
Catalog No.:BCC8746
CAS No.:66335-38-4
- Dihydroguaiaretic acid
Catalog No.:BCN4212
CAS No.:66322-34-7
- ML 171
Catalog No.:BCC6252
CAS No.:6631-94-3
- 3H-1,2-Benzodithiol-3-one-1,1-dioxide
Catalog No.:BCC8633
CAS No.:66304-01-6
- Pregomisin
Catalog No.:BCN7000
CAS No.:66280-26-0
- Gomisin J
Catalog No.:BCN2270
CAS No.:66280-25-9
- 4-Amino-2-methylquinoline
Catalog No.:BCC8677
CAS No.:6628-04-2
- 6'-O-Acetylpaniculoside II
Catalog No.:BCC8316
CAS No.:
- Stemonidine
Catalog No.:BCC8253
CAS No.:66267-46-7
- N-dotriacontanol
Catalog No.:BCC8218
CAS No.:6624-79-9
- N-Benzylanthranilic acid
Catalog No.:BCC9093
CAS No.:6622-55-5
- TGX-221
Catalog No.:BCC1244
CAS No.:663619-89-4
- Methylsyringol
Catalog No.:BCN3535
CAS No.:6638-05-7
- (+)-Apogossypol
Catalog No.:BCC5585
CAS No.:66389-74-0
- Chloranthalactone A
Catalog No.:BCN8022
CAS No.:66395-02-6
- Chloranthalactone B
Catalog No.:BCN8020
CAS No.:66395-03-7
- 5alpha-Hydroxychloranthalactone A
Catalog No.:BCN7469
CAS No.:66395-04-8
- Pamoic acid disodium salt
Catalog No.:BCC7909
CAS No.:6640-22-8
- 13-Hydroxyoxyberberine
Catalog No.:BCN3355
CAS No.:66408-27-3
- 6-Amino-1,3-dimethyluracil
Catalog No.:BCC8755
CAS No.:6642-31-5
- 2-(2,4-Diaminophenoxy)ethanol dihydrochloride
Catalog No.:BCN8497
CAS No.:66422-95-5
- Kaempferol 3-O-(6''-O-acetyl)glucoside-7-O-rhamnoside
Catalog No.:BCN1385
CAS No.:66465-24-5
- H-D-Lys(Boc)-OMe.HCl
Catalog No.:BCC2990
CAS No.:66494-53-9
Formulation and Taste Masking of Ranitidine Orally Disintegrating Tablet.[Pubmed:28243264]
Iran J Pharm Res. 2016 Fall;15(4):677-686.
Orally Disintegrating Tablets (ODT) have the advantages of both solid dosage form specially the stability and ease of handling and liquid dosage forms including ease of swallowing and pre-gastric absorption. We focused on taste masking and formulation of ranitidine ODT which disintegrates rapidly in the mouth within 60 sec using super-disintegrants, special polymers, water soluble and even insoluble excipients, sweeteners and essence. Various formulations were designed and made in four series. The amount of ranitidine in each formulation was 150 mg, and the final weight of tablets was around 500 mg. Prepared formulations were evaluated in terms of several physicochemical tests including powder/granule flowability, appearance, thickness, uniformity of weight, hardness, friability and disintegration time. Several taste masking techniques were investigated in each series of formulation, in order to cover the bitter taste of wranitidine. These included the addition of sweetener, granulation, solid dispersion with soluble and insoluble agents and complex formation with cellulose derivatives. The best formulation(s) in each group was/were chosen for taste evaluations with the help of 10 volunteers. Finally, formulation F14 was selected as the ultimate formulation, based on its better taste and shorter disintegration time (around 5 seconds). Formulation F14 contained Na CMC, avicel, Na starch glycolate, xylitol, saccharin, Na benzoate and menthol. The chosen formulation successfully passed the complementary evaluations such as assay of active ingredient and dissolution time. Na CMC was found to be acceptable in terms of decreasing disintegration time and enhanced taste masking potential and can be used in further ODT formulations.
Downbeat nystagmus due to ranitidine in a pediatric patient.[Pubmed:28284887]
Eur J Paediatr Neurol. 2017 Jul;21(4):682-684.
BACKGROUND: Ranitidine has not been considered as a potential cause of ocular movement conditions. However, it is known that the vestibular nucleus complex, that has a key role in gaze control and vestibule-ocular reflexes, receives hypothalamic histaminergic innervations. Some studies reported the effect of ranitidine blocking the excitatory responses of vestibular nuclei neurons to histamine. CASE REPORT: We report the first case of a downbeat nystagmus secondary to ranitidine in an infant. A 3-month-old female developed a downbeat gaze after starting treatment with ranitidine for a pediatric gastroesophageal reflux disease. Microbiological test were negative and neuroblastoma evaluation was normal. CONCLUSION: As ranitidine is widely prescribed in the pediatric population, clinicians should be aware of its potential to cause ocular movements disorders.
Ranitidine: forgotten drug of delayed gastric emptying.[Pubmed:28159802]
BMJ Support Palliat Care. 2017 Sep;7(3):255-257.
Delayed gastric emptying in the presence or absence of mechanical bowel obstruction can cause distressing symptoms in palliative care patients. We present two patients, both with vomiting due to delayed gastric emptying and gastric outlet obstruction secondary to pancreatic cancer, treated with subcutaneous ranitidine resulting in a symptomatic response. We hypothesise that ranitidine is a useful adjunct to standard treatment with prokinetic agents or octreotide in such patients and potentially those with proximal mechanical bowel obstruction from other malignancies with associated delayed gastric emptying.
Optimization and Evaluation of Gastroretentive Ranitidine HCl Microspheres by Using Factorial Design with Improved Bioavailability and Mucosal Integrity in Ulcer Model.[Pubmed:28271373]
AAPS PharmSciTech. 2017 May;18(4):957-973.
The purpose of our investigation was to develop and optimize the drug entrapment efficiency and bioadhesion properties of mucoadhesive chitosan microspheres containing ranitidine HCl prepared by an ionotropic gelation method as a gastroretentive delivery system; thus, we improved their protective and therapeutic gastric effects in an ulcer model. A 3 x 2(2) full factorial design was adopted to study the effect of three different factors, i.e., the type of polymer at three levels (chitosan, chitosan/hydroxypropylmethylcellulose, and chitosan/methylcellulose), the type of solvent at two levels (acetic acid and lactic acid), and the type of chitosan at two levels (low molecular weight (LMW) and high molecular weight (HMW)). The studied responses were particle size, swelling index, drug entrapment efficiency, bioadhesion (as determined by wash-off and rinsing tests), and T 80% of drug release. Studies of the in vivo mucoadhesion and in vivo protective and healing effects of the optimized formula against gastric ulcers were carried out using albino rats (with induced gastric ulceration) and were compared to the effects of free ranitidine powder. A pharmacokinetic study in rabbits showed a significant, 2.1-fold increase in theAUC0-24of the ranitidine microspheres compared to free ranitidine after oral administration. The optimized formula showed higher drug entrapment efficiency and mucoadhesion properties and had more protective and healing effects on induced gastric ulcers in rats than ranitidine powder. In conclusion, the prolonged gastrointestinal residence time and the stability of the mucoadhesive microspheres of ranitidine as well as the synergistic healing effect of chitosan could contribute to increasing the potential of its anti-gastric ulcer activity.
Some in vitro and in vivo actions of the new histamine H2-receptor antagonist, ranitidine.[Pubmed:6112034]
Br J Pharmacol. 1981 Jan;72(1):49-54.
1 Ranitidine has been investigated as an antagonist of the H2-receptor-mediated responses to histamine of guniea-pig atrium and rat uterus in vitro and as an inhibitor of gastric acid secretion in the rat. 2 Ranitidine competitively antagonized histamine-induced increases in contraction frequency of the guinea-pig isolated rat atrium. Ranitidine had a pA2 of 7.2 and was 7.9 and 4.5 times more potent than metiamide and cimetidine respectively. 3 Ranitidine competitively antagonized histamine-induced relaxations of the rat isolated uterine horn. Ranitidine had a pA2 of 6.95 and was 3.6 and 5.9 times more potent than metiamide and cimetidine respectively. 4 Ranitidine, even at high concentrations, did not affect responses of the guinea-pig isolated atrium or rat isolated uterus to (-)-isoprenaline. Similarly it was without effect on either histamine or bethanechol-induced contractions of guinea-pig isolated ileum. 5 Ranitidine inhibited histamine- and pentagastrin-induced gastric acid secretion in the perfused stomach preparation of the anaesthetized rat. Ranitidine was 5.2 and 7.0 times more potent on a molar basis than metiamide and cimetidine respectively, as an inhibitor of histamine-induced gastric acid secretion. 6 It is concluded that ranitidine is a potent, competitive and selective antagonist of histamine at H2-receptor sites and and effective inhibitor of gastric acid secretion in vivo.
Antagonism of vasodepressor and gastric secretory responses to histamine by the H2-receptor antagonists, ranitidine and cimetidine, in the anaesthetized dog.[Pubmed:6112035]
Br J Pharmacol. 1981 Jan;72(1):55-60.
1 The new H2-receptor antagonist, ranitidine, has been compared with cimetidine as an inhibitor of gastric acid secretion in the anaesthetized dog. 2 Both ranitidine and cimetidine given intravenously inhibited histamine-induced gastric secretion in a dose-related manner. Ranitidine was 4.2 times more potent than cimetidine when given as an intravenous infusion and 9.6 times more potent as an intravenous bolus dose. 3 In a separate series of experiments, ranitidine was compared with cimetidine as an antagonist of vasodepressor responses to histamine. Mepyramine alone displaced the histamine dose-response curve to the right. After a maximally effective dose of mepyramine, further displacement could be achieved with ranitidine and cimetidine, ranitidine being 19.2 times more potent than cimetidine. 4 Ranitidine alone has no effect on vasodepressor response curves to histamine, acetylcholine or (-)-isoprenaline or on vasopressor response curves to phenylephrine. 5 These results indicate that displacement of the histamine dose-response curve after mepyramine blockade by ranitidine and cimetidine is due to selective H2-receptor antagonism.


